Canine dermatomyositis (DM) is an inflammatory ischemic vasculopathy of immune origin with a strong genetic predisposition, primarily affecting the skin and, to a lesser extent, striated muscles. We present a comprehensive pathogenic, diagnostic, and therapeutic overview of this dermatosis.
June 2025
Introduction
Initially recognized in Collies and Shetland Sheepdogs, this condition serves as a particularly relevant spontaneous model for the study of human juvenile dermatomyositis (JDM), a systemic autoimmune disease with striking clinical and histopathological similarities. Canine DM is therefore not only a clinical concern for veterinary practitioners but also a valuable field for translational research.
Over the last decade, the understanding of canine DM has undergone a radical transformation. The concept of a simple hereditary disease with dominant autosomal transmission, variable penetrance, and inconsistent expressivity has been superseded by a much more nuanced model of a complex genetic disorder. Advances in genomics have allowed for the identification of specific risk loci and their epistatic interactions, providing a predictive framework for the disease. Concurrently, fundamental research has highlighted the central role of dysregulation of the type I interferon (IFN) signaling pathway as a major pathogenic driver, similar to what is observed in human JDM. This convergence of genetic and immunopathological knowledge has paved the way for more targeted diagnostic and therapeutic approaches that redefine the management of this disease in 2025. We present an up-to-date overview of canine dermatomyositis, incorporating the latest discoveries on its pathogenesis, diagnosis, and emerging therapeutic strategies.
Etiopathogenesis
The etiopathogenesis of canine dermatomyositis is the result of a complex interaction between polygenic genetic predisposition, dysregulation of the innate immune system, and environmental triggers. Understanding this cascade of events, from gene to lesion, is fundamental to comprehending the disease and justifying modern therapeutic approaches.
1.1 The Genetic Component of Canine Dermatomyositis
The historical notion of autosomal dominant inheritance with incomplete penetrance has been refined by genome-wide association studies (GWAS) that have revealed a complex and epistatic inheritance pattern. The risk of developing DM in predisposed breeds, such as the Collie and Shetland Sheepdog, is now understood to be determined by the interaction of variants within three independent genetic loci.
Identification of Risk Loci
Research has identified three major chromosomal regions whose specific alleles modulate the risk of DM.
- Locus A (Chromosome 10): A very strong association has been found with a missense mutation in the PAN2 gene. The PAN2 gene codes for a subunit of the poly(A) nuclease deadenylation complex, which plays a crucial role in regulating messenger RNA (mRNA) degradation. This function is particularly important for regulating the inflammatory response, notably through the stabilization of pro-inflammatory gene transcripts containing adenine-uracil-rich elements (AREs), such as interleukin 6 (IL-6), a cytokine known to be overexpressed in human JDM. This mutation therefore establishes a direct link between a fundamental genetic defect and a predisposition to immune dysregulation.
- Locus B (Chromosome 31): A second significant association has been identified with an insertion/deletion (indel) in a non-coding 5′ region of the MAP3K7CL gene. Although the precise function of this gene, which codes for a kinase, is still poorly studied, it is primarily expressed in peripheral blood leukocytes, suggesting a role in immune function.
- Locus C (Canine MHC): A significant association has been observed with a specific haplotype of the canine major histocompatibility complex (MHC) class II. The association is particularly strong in individuals homozygous for this haplotype. However, the high frequency of this risk haplotype in healthy dogs indicates that it is not sufficient on its own to cause the disease and that its influence is modulated by the other risk loci.
Epistatic Interaction and Risk Stratification
Combined analysis of the genotypes of these three loci revealed a risk pattern that is not simply additive but epistatic, where the effect of one gene is modified by the presence of one or more other genes. Research has shown that 9 of the 27 possible genotypic combinations confer a moderate (33-50% penetrance) or high (90-100% penetrance) risk of developing DM, thus explaining 93% of the cases studied. For example, genotypes AABB (homozygous for risk alleles at loci A and B) show 100% penetrance when associated with at least one risk allele at locus C (AABBCc or AABBCC).
This discovery transforms the understanding of the disease. The old concepts of “incomplete penetrance” and “variable expressivity” can now be reinterpreted in light of this quantitative genetic model. These phenotypic variations are not random but are a direct reflection of the polygenic risk profile of each individual. A dog with a high-risk genotype is almost certain to develop the disease if exposed to appropriate triggers, while a dog with a moderate-risk genotype may never express the disease or show only mild and transient signs. Genetics thus provides a predictive framework that replaces uncertainty. This has profound implications for genetic counseling, which shifts from a simple recommendation not to breed clinically affected dogs to a proactive selection strategy for breeding pairs aimed at minimizing the production of puppies carrying high-risk genotypic combinations.
Genotype-Phenotype Correlation
A significant inverse correlation has been established between the number of risk alleles at the PAN2 and MAP3K7CL loci and the age of disease onset. Dogs carrying four risk alleles (homozygous for risk at both loci) develop DM at a median age of 5 months, while those with only two risk alleles develop it at a median age of 18.5 months. This correlation suggests a gene dose effect on susceptibility severity, where a higher genetic load requires a lower environmental trigger threshold to initiate the disease.
Table 1: Genetic Risk Loci for Canine Dermatomyositis
|
Locus Name |
Associated Gene |
Chromosome |
Normal Allele (notation) |
Known/Hypothetical Biological Function |
|---|---|---|---|---|
|
Locus A |
PAN2 |
10 |
Wildtype (a) |
Regulation of mRNA degradation, stabilization of inflammatory response transcripts |
|
Locus B |
MAP3K7CL |
31 |
Wildtype (b) |
Kinase expressed in immunological tissues, potential role in immune signaling |
|
Locus C |
DLA-DRB1 |
12 (MHC) |
Other DLA haplotypes (c) |
Antigen presentation, regulation of adaptive immune response |
Table 2: DM Risk Stratification Based on Genotype (Combinations of Loci A and B, modified by Locus C)
|
Locus A / Locus B Genotype |
Risk with Locus C = cc |
Risk with Locus C = Cc |
Risk with Locus C = CC |
|---|---|---|---|
|
aa bb |
Low |
Low |
Low |
|
Aa bb |
Low |
Low |
Low |
|
aa Bb |
Low |
Low |
Low |
|
AA bb |
Unknown |
Moderate |
Moderate |
|
Aa Bb |
Low |
Low |
Low |
|
aa BB |
Low |
Moderate |
Moderate |
|
AA Bb |
Unknown |
Moderate |
High |
|
Aa BB |
Unknown |
Moderate |
High |
|
AA BB |
Unknown |
High |
High |
|
Table adapted from data from the UC Davis Veterinary Genetics Laboratory, based on research by Evans et al. |
|||
1.2 The Cascade of Immune Dysregulation: Vasculopathy and Interferon
Genetic predisposition is only the first step. The pathogenesis of DM is fundamentally a disease of the immune system that targets blood vessels, leading to tissue ischemia.
Ischemic Vasculopathy as a Primary Lesion
DM is primarily an inflammatory vasculopathy. The central pathological process is an immune-mediated attack directed against the endothelium of small blood vessels, particularly endomysial capillaries in muscles and dermal vessels in the skin. This endothelial inflammation, or endotheliitis, leads to narrowing of the vascular lumen, microthrombosis, and, ultimately, hypoperfusion of the downstream tissues. The resulting ischemia is responsible for the characteristic clinical manifestations: atrophy and necrosis of hair follicles and the epidermis (causing alopecia and ulceration) and degeneration of muscle fibers (causing atrophy and weakness).
Type I Interferon (IFN) as a Central Pathogenic Driver
Overwhelming evidence, from human and canine models, indicates that hyperactivation of the type I interferon signaling pathway is the primary driver of this vasculopathy. This “IFN signature” manifests as systemic and tissue (skin, muscle, blood) overexpression of a set of genes whose transcription is induced by IFN, such as MXA, ISG15, and CXCL10. The strength of this signature in the skin is directly correlated with skin disease activity and decreases or disappears in patients in remission, making it a potential biomarker of disease activity. Type I interferons have pleiotropic effects that can explain vasculopathy: they can activate endothelial cells, promote a pro-inflammatory and pro-thrombotic state, and recruit immune cells to the site of inflammation, thus perpetuating vascular damage.
Role of Autoantibodies and Circulating Immune Complexes (CICs)
Although the search for specific myositis autoantibodies is less advanced in dogs than in humans, older studies have established a positive correlation between the clinical severity of canine DM and serum levels of circulating immune complexes (CICs) and immunoglobulin G (IgG). It is postulated that these CICs can deposit in the microvessel wall, activate the complement cascade, and thus contribute to endotheliitis and vasculopathy.
The convergence of these data allows for the establishment of a unified causal chain that was not evident a decade ago. First, a genetic predisposition, particularly through mutations in inflammatory regulatory genes such as PAN2, creates fertile ground for an aberrant immune response. Second, in the presence of triggers, this predisposition leads to excessive and sustained production of type I IFN, the central mechanism of the disease. Third, this IFN-rich environment induces vasculopathy by directly damaging endothelial cells. Fourth, vasculopathy compromises blood supply, causing ischemia in vulnerable tissues. Finally, this ischemia clinically manifests as the characteristic skin and muscle lesions of DM. This unified understanding of pathogenesis fully justifies the current paradigm shift in therapy, moving away from general immunosuppressants towards therapies that specifically target and block the IFN signaling pathway.
1.3 Environmental and Endogenous Triggers
In a genetically predisposed individual, triggering factors are often necessary to initiate or exacerbate the disease.
- Documented Risk Factors: Several factors are known to play a role in triggering clinical flare-ups.
-
- Mechanical Trauma: The typical location of lesions on bony prominences (face, carpi, tarsi) and pressure points strongly suggests that repeated microtrauma acts as a local trigger, a Koebner phenomenon.
- Exposure to Ultraviolet (UV) Rays: The photodistribution of certain facial lesions and reported exacerbation after sun exposure implicate UV as an important triggering factor, probably by inducing keratinocyte apoptosis and releasing autoantigens.
- Hormonal Factors: Disease flare-ups have been associated with estrus, suggesting a hormonal influence on immune regulation.
- The Role of Vaccination: The link between vaccination and DM is an important topic of discussion. There is a well-recognized syndrome of post-vaccinal ischemic dermatopathy, particularly after rabies vaccination, which is clinically and histologically indistinguishable from DM. A retrospective study of 177 cases of ischemic dermatopathy estimated that an association with recent vaccination was probable in 48.3% of cases. Rather than considering post-vaccinal dermatopathy as an entirely distinct entity, it is more logical to view it as genetic DM unmasked by the powerful immune stimulus that vaccination represents. For a puppy carrying a high-risk genotype (e.g., AABB), the intense immune stimulation induced by a vaccine could be the “stress test” sufficient to initiate the pathogenic IFN cascade and trigger clinical disease. This has important clinical implications: for puppies of at-risk breeds with an unknown or high-risk genetic status, it is not about avoiding vaccination, but about informing owners and implementing close monitoring to detect the appearance of skin lesions in the weeks and months following injections, to allow for early intervention.
Clinical Spectrum and Diagnostic Approach
The clinical presentation of canine DM is variable, ranging from mild and self-limiting cutaneous forms to severe systemic manifestations. Diagnosis relies on a multimodal approach combining signalment, clinical signs, and targeted complementary examinations.
2.1 Breed Predisposition and Disease Classification
- Familial DM: The Collie and Shetland Sheepdog are emblematic breeds for the disease, in which a hereditary basis has been proven and genetic risk loci have been identified.
- DM-like Syndromes: Similar clinical and histological pictures have been described in a growing number of other breeds, including the Beauceron, Welsh Corgi, Lakeland Terrier, Chow Chow, German Shepherd, and Kuvasz.
The historical distinction between “familial DM” (in predisposed breeds) and “DM-like” (in other breeds) is increasingly considered semantic rather than biological. The underlying histopathology is identical, suggesting common pathogenic pathways. The term “ischemic dermatopathy” is a more precise and unifying histopathological diagnosis that encompasses these different clinical presentations. The modern diagnostic approach should therefore not stop at breed, but rather seek genetic and pathological markers of the disease in any dog presenting a compatible clinical picture, regardless of its breed.
2.2 Clinical Manifestations
The clinical signs of DM vary considerably from individual to individual but generally follow a recognizable pattern.
Cutaneous Signs
Cutaneous involvement is the most constant manifestation of the disease.
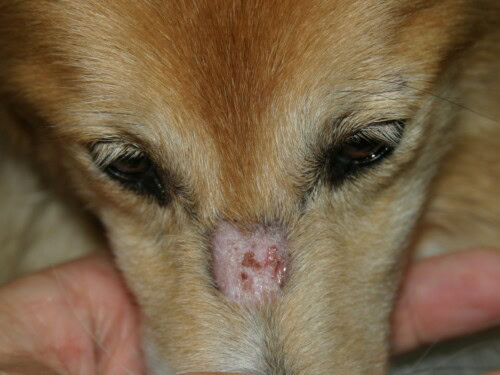
- Initial Lesions: They typically appear in young dogs, before 6 months of age, and often as early as 7 to 11 weeks. The initial presentation can be subtle, with transient papules, pustules, or vesicles that rapidly evolve into more characteristic lesions of erythema, alopecia, scaling, and crusting.
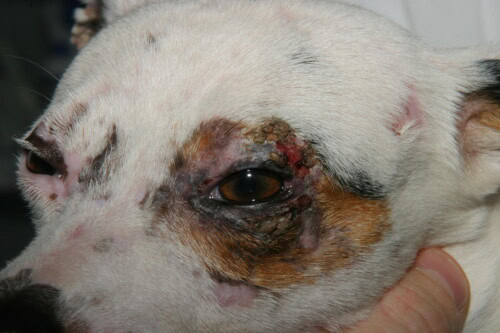
- Characteristic Distribution: Lesions have a predilection for areas of trauma and poor collateral circulation: the face (periorbital and perioral regions, muzzle), ear pinnae (especially the tip and folds), tail tip, and skin over bony prominences of the limbs (carpi, tarsi, digits).
- Chronic Lesions: Over time, the lesions evolve into permanent cicatricial alopecia, cutaneous atrophy, and pigmentary changes (hyperpigmentation or hypopigmentation), creating a mottled appearance called poikiloderma. Claw involvement (onychodytrophy, onychorrhexis, onychoschizia) may also be observed.
Severe tail involvement with alopecia and crusted lesions
Mainly unilateral palpebral involvement in this dog
Lesion that can perfectly mimic dermatophytosis
Myopathic Signs
Muscle involvement is more variable, and when clinically apparent, it is generally a sign of a more severe form of the disease.
- Correlation with Cutaneous Signs: Clinical myositis usually occurs after the onset of skin lesions, and its severity is often proportional to that of the dermatological involvement.
- Muscle Atrophy: Atrophy of the masticatory muscles (temporalis and masseter) is the most frequent muscular sign and can be the only myopathic manifestation. In more severe cases, bilaterally symmetrical atrophy of the muscles of the head, neck, trunk, and limbs can develop.
- Functional Signs: Atrophy and inflammation of the muscles of mastication and swallowing can lead to difficulty grasping food (prehension), chewing, and swallowing (dysphagia). An abnormal, stiff, and stilted gait (“stiff-stilted gait” or “goose step”) is also described.
- Severe Complications: The development of megaesophagus (dilatation and hypomotility of the esophagus) is a serious complication, leading to regurgitation, weight loss, and a high risk of aspiration pneumonia, a frequent cause of mortality in severe forms of DM.
2.3 Definitive Diagnosis: A Multimodal Strategy
The diagnosis of DM is never based on a single test, but on the convergence of several elements, after excluding common differential diagnoses such as demodicosis, bacterial pyoderma, dermatophytosis, and discoid lupus erythematosus.
- Histopathology (Cornerstone): Biopsy is the key examination to confirm the ischemic nature of the lesions.
-
- Cutaneous Biopsy: Taken from a recent and active lesion, it reveals characteristic changes of ischemic dermatopathy: a paucicellular interface dermatitis (with few inflammatory cells), atrophy of hair follicles, vacuolization of keratinocytes in the basal layer of the epidermis, the presence of apoptotic cells (Civatte bodies), and fibrosis or mucin accumulation in the dermis. Vascular changes can be subtle, manifesting as a “fuzzy” and eosinophilic appearance of perivascular collagen (“smudged collagen”) rather than obvious necrotizing vasculitis.
- Muscle Biopsy: Although less systematically performed in routine practice, it is indicated in cases of suspected myopathic involvement. It typically shows interstitial and perivascular myositis, predominantly lymphocytic, plasmacytic, and histiocytic, associated with signs of muscle fiber distress (degeneration, necrosis, regeneration, and atrophy).
- Electrodiagnostic (EMG): Electromyography is a very sensitive tool for detecting myopathy, even in the absence of obvious clinical signs. The examination, performed under anesthesia, reveals abnormal spontaneous electrical activities in the affected muscles, such as fibrillation potentials, positive sharp waves, and bizarre high-frequency or myotonic discharges.
- Genetic Tests: For Collies, Shetland Sheepdogs, and their crosses, testing of the three risk loci (PAN2, MAP3K7CL, DLA) has become an essential diagnostic and prognostic tool. It allows for confirmation of genetic predisposition and stratification of the animal’s risk (low, moderate, or high), which helps guide owner counseling.
- Clinical Biochemistry: Measurement of serum creatine kinase (CK) is useful. A marked elevation is an indicator of active myositis and muscle damage. However, a normal CK value does not rule out the diagnosis, as muscle involvement can be minimal, focal, or chronic with little active inflammation.
Table 3: Diagnostic Modalities for Canine Dermatomyositis
|
Diagnostic Modality |
Expected Results / Key Abnormalities |
|---|---|
|
Cutaneous Histopathology |
Paucicellular interface dermatitis, follicular atrophy, basal vacuolization, Civatte bodies, dermal fibrosis/mucinosis, “smudged collagen” |
|
Muscle Histopathology |
Interstitial and perivascular myositis (lympho-plasmacytic), degeneration/regeneration/atrophy of muscle fibers |
|
Electromyography (EMG) |
Abnormal spontaneous activity: fibrillation potentials, positive sharp waves, myotonic/bizarre high-frequency discharges |
|
Genetic Test (3 loci) |
Identification of risk alleles at PAN2, MAP3K7CL, and DLA loci; calculation of a risk score (Low, Moderate, High) |
|
Biochemistry (Creatine Kinase) |
Elevation (often marked) in active myositis, but may be normal in chronic forms or with low muscle involvement |
Therapeutic Strategies and Management in 2025
Prognosis and Long-Term Outcome
The prognosis for canine DM is highly variable and depends on a multitude of factors. Understanding these indicators and the disease’s evolutionary profiles is essential for providing owners with realistic expectations and for adapting long-term management.
4.1 Prognostic Indicators: Predicting the Future
Several factors allow for estimation of the probable course of the disease in a given dog.
- Clinical Factors:
-
- Disease Severity: This is the most direct prognostic factor. Mild cases, with only a few non-ulcerated skin lesions, have an excellent prognosis and may even go into spontaneous remission without sequelae. Moderate cases have a good prognosis for control, but often with residual cicatricial alopecia. Severe cases, with extensive ulcerations and/or significant muscle involvement, have a guarded to poor prognosis.
- Muscle and Esophageal Involvement: The presence of clinically significant myopathy, and especially the development of megaesophagus, significantly worsens the prognosis. The risk of malnutrition, mis-swallowing, and aspiration pneumonia becomes a major life-threatening concern.
- Demographic Factors: A retrospective study of 177 cases of ischemic dermatopathy (excluding classic familial DM) identified two factors associated with a poorer outcome: body weight less than 10 kg and older age at the time of biopsy.
- Genetic Factor: The integration of the genetic risk profile (based on the 3 loci) into prognostic evaluation represents a major advance. This test allows for a shift from a reactive prognosis, based on already present clinical signs, to a proactive and predictive prognosis. A Collie or Shetland puppy tested at birth and revealing a high-risk genotype (e.g., AABBCC) has an intrinsically more reserved prognosis, even before the appearance of any clinical signs. It is predicted that it will develop the disease earlier and potentially in a more severe form. This information allows for early stratification of patients and the implementation of reinforced monitoring and an aggressive therapeutic intervention strategy at the first signs, which could improve the long-term outcome.
4.2 Disease Course and Long-Term Management
By analogy with human JDM, canine DM can follow different evolutionary patterns over time.
- Evolutionary Profiles:
-
- Monocyclic: The disease manifests as a single episode, often in mildly affected animals, which enters complete remission (sometimes spontaneously) and does not recur. The prognosis is excellent.
- Polycyclic: The disease evolves in flare-ups, with periods of remission interspersed with relapses. These flare-ups are often triggered by factors (UV exposure, trauma, estrus). Management aims to control flare-ups and extend remission periods.
- Chronic-Continuous: The disease remains persistently active, without periods of complete remission. These cases require lifelong treatment and are associated with the most guarded prognosis.
- Chronic Management: Managing chronic forms (polycyclic or continuous) is a challenge. The goal is to keep the disease under control with the lowest effective dose of medication to limit cumulative side effects. The use of steroid-sparing agents (like cyclosporine) and, increasingly, targeted therapies like JAK inhibitors, is central to this strategy. Regular clinical and biological monitoring is essential to adjust treatment and monitor for the occurrence of complications, including a potentially increased risk of kidney disease in dogs suffering from long-term chronic inflammation.
Conclusion
In 2025, canine dermatomyositis has evolved from an enigmatic hereditary disease to a complex genetic disorder whose molecular mechanisms are increasingly well understood. The recognition of its etiopathogenesis, centered on an ischemic vasculopathy driven by dysregulation of the type I interferon pathway and underpinned by specific genetic interactions, has transformed its clinical approach.
This paradigm shift has profound and concrete implications. Diagnosis has evolved to integrate genetic testing as an essential pillar alongside histopathology and electromyography, allowing for early risk stratification and more precise prognosis. On the therapeutic front, the arsenal has expanded, moving from broad-spectrum immunosuppressants, effective but burdened with side effects, to targeted therapies that specifically block identified pathogenic pathways. The use of Janus Kinase inhibitors, in particular, represents the new frontier of treatment, offering hope for more effective and safer disease control.
Future research perspectives are clear. They will need to focus on identifying risk loci in other canine breeds, large-scale validation of the efficacy and long-term safety of targeted therapies through randomized clinical trials, and the development of blood biomarkers (such as the IFN signature) for non-invasive monitoring of disease activity and increased personalization of treatment. The canine dermatomyositis model, due to its relevance and complexity, will continue to be an invaluable resource for advancing the understanding and treatment of this debilitating disease, both for the well-being of dogs and for the knowledge it provides to human medicine.
References
- Evans, J. M., Noorai, R. E., Tsai, K. L., Starr-Moss, A. N., Hill, C. M., Anderson, K. J., Famula, T. R., & Clark, L. A. (2017). Beyond the MHC: A canine model of dermatomyositis shows a complex pattern of genetic risk involving novel loci. PLOS Genetics, 13(2), e1006604.
- Hargis, A. M., Prieur, D. J., Haupt, K. H., Collier, L. L., & Evermann, J. F. (1985). Familial canine dermatomyositis. Initial characterization of the cutaneous and muscular lesions. American Journal of Pathology, 121(3), 521–530.
- Rees, C. A., & Boothe, D. M. (2003). Therapeutic response to pentoxifylline and its active metabolites in dogs with familial canine dermatomyositis. Veterinary Therapeutics: Research in Applied Veterinary Medicine, 4(3), 234–241.
- Hargis, A. M., Prieur, D. J., Haupt, K. H., McDonald, T. L., & Moore, M. P. (1986). Prospective study of familial canine dermatomyositis. Correlation of the severity of dermatomyositis and circulating immune complex levels. American Journal of Pathology, 123(3), 465–479.
- Hargis, A. M., & Ginn, P. E. (2001). Canine inflammatory myopathies: a clinicopathologic review of 200 cases. Veterinary Pathology, 38(6), 688-699.
- Hargis, A. M., Haupt, K. H., Hegreberg, G. A., Prieur, D. J., & Moore, M. P. (1984). Familial canine dermatomyositis. Initial characterization of the cutaneous and muscular lesions. American Journal of Pathology, 116(2), 234–244.
- Hargis, A. M., & Prieur, D. J. (1987). Prospective study of familial canine dermatomyositis. Correlation of the severity of dermatomyositis and circulating immune complexes. American Journal of Pathology, 128(1), 129–139.
- Pumphrey, S. A., & Outerbridge, C. A. (2019). The role of oclacitinib in the management of ischaemic dermatopathy in four dogs. Veterinary Dermatology, 30(4), 346-e100.
- Mandrioli, L., & Biserni, R. (2024). A case of refractory nasal dermatomyositis-like disease in an adult Poodle. ResearchGate.
- Errante, P. R. (2022). Dermatomyositis Disease in Dogs. International Journal of Zoology and Animal Biology, 5(8).
- Mendoza, F., & Toral, M. (2019). Three Cases of Canine Dermatomyositis-Like Disease. Acta Scientiae Veterinariae, 47, 1632.
- Mellett, M., & Allen, A. G. (2018). Dermatomyositis etiopathogenesis: a rebel soldier in the muscle. Current Opinion in Rheumatology, 30(6), 610–616.
- Marie, I., Drouot, L., Menard, J. F., & Levesque, H. (2006). Long-term outcome and prognostic factors of juvenile dermatomyositis: a multinational, multicenter study of 490 patients. Arthritis and Rheumatism, 54(11), 3683–3692.
- Kmieć, P., & Gomułkiewicz, A. (2021). The role of interferons type I, II and III in myositis: A review. Clinical and Experimental Rheumatology, 39(4), 882-890.
- Greenberg, S. A., & Pinkus, J. L. (2011). A broad molecular profile of dermatomyositis skin reveals prominent interferon-inducible gene expression and demonstrates molecular similarity to lupus skin. PLoS ONE, 6(12), e29161.
- Berger, D. (2016). Canine Dermatomyositis. Clinician’s Brief, 14(11), 50-52.
- Ghafour, A., & Isenberg, D. A. (2020). The immunopathogenesis of dermatomyositis. Best Practice & Research Clinical Rheumatology, 34(4), 101533.
- Mandell, D. C., & Dehghanpir, S. D. (2021). An update on the diagnosis and treatment of inflammatory myopathies. Journal of Clinical Medicine, 10(15), 3381.
- Main-Nolton, A., & Clark, L. A. (2022). Transcriptome Profiling of Canine Familial Dermatomyositis (DMS) Skin Lesions and Treatment in Collies and Shetland sheepdogs. VIN.
- Santoro, D., & Pucheu-Haston, C. M. (2014). Effects of pentoxifylline on in vivo and in vitro IgE-mediated mast cell degranulation and cutaneous inflammation in dogs. American Journal of Veterinary Research, 75(2), 152–158.
- Olivry, T., & Banovic, F. (2019). Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). Veterinary Dermatology, 30(3), 169-e50.
- Cosgrove, S. B., Cleaver, D. M., King, V. L., Gilmer, A. R., Daniels, A. E., Wren, J. A., & Stegemann, M. R. (2013). A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Veterinary Dermatology, 25(2), 91-e23.
- Gortel, K. (2006). What’s new in dermatologic therapy? DVM360.
- Rees, C. A., & Boothe, D. M. (2004). Therapeutic response to pentoxifylline and its active metabolites in dogs with familial canine dermatomyositis. Veterinary Therapeutics: Research in Applied Applied Veterinary Medicine, 4(3), 234-241.
- Archer, T. M., & Boothe, D. M. (2004). Cyclosporine: a review of its use in veterinary dermatology. Veterinary Dermatology, 15(6), 349–361.
- Robson, D. C. (2014). Cyclosporine in Veterinary Dermatology. Proceedings of the 39th World Small Animal Veterinary Association World Congress.
- Alexishcheva, A., & Guryeva, S. (2024). Interferon signature as a biomarker of skin disease activity in patients with refractory juvenile dermatomyositis. Frontiers in Medicine, 11, 1214920.
- Navale, S., & De, A. (2024). Clinical Progress in Mesenchymal Stem Cell Therapy: A Focus on Rheumatic Diseases. ResearchGate.
- de Mello, M. F., & Hagiwara, M. K. (2007). Canine ischaemic dermatopathy: a retrospective study of 177 cases (2005-2016). Veterinary Dermatology, 28(5), 485-e117.
- Gury, T., & Seror, R. (2024). Prognostic factors and long-term outcome in dogs diagnosed with primary and secondary immune thrombocytopenia in Ireland. Journal of Small Animal Practice.
- Fantini, D., & Ciliberto, G. (2022). Novel Management of Masticatory Myositis in Three Dogs with a Selective Janus Kinase (JAK)-1 Inhibitor. ResearchGate.
- Masi, G., & D’Alessandro, A. (2024). Recent advances in research on dermatomyositis. Uptocure.
- Ardalan, K., & Marques, M. C. (2025). Psychometric properties of patient-reported outcomes measurement information system (PROMIS) fixed short forms in Juvenile Myositis. Seminars in Arthritis and Rheumatism, 71.
- Bartels, C., & Putterman, C. (2022). Juvenile dermatomyositis: updates in pathogenesis and biomarkers. Current Rheumatology Reports, 24(10), 307-317.
- Ziskin, J., & Fiorentino, D. (2024). Recent advances in the treatment of dermatomyositis. Current Opinion in Rheumatology, 36(3), 203-210.
- Mandl, T., & Lundberg, I. E. (2019). The role of interferons type I, II and III in myositis: A review. Clinical and Experimental Rheumatology, 37(5), 868-877.
- van der Kooi, A. J., & van de Vlekkert, J. (2023). An inflammatory myopathy in the Dutch Kooiker dog: a study of 119 cases. Journal of Veterinary Internal Medicine, 37(2), 643-653.
- Viviano, K. R. (2016). Cyclosporine: a review of its use in veterinary medicine. Veterinary Clinics of North America: Small Animal Practice, 46(2), 245-265.
- Nuttall, T., & Gey, van Pittius, M. (2023). Immunosuppressant Therapy in Small Animal Medicine. Today’s Veterinary Practice.
Related Searches
canine dermatomyositis, dermatomyositis, veterinary clinic, dog, case, in dogs, disease, chow chow, breeds, clinical signs, treatment, dermatology, shetland, familial dermatomyositis, skin, dermatosis, collie, muscles, éric guaguère, dermatomyositis in, symptoms, emmanuel bensignor, redness, consultation, cause, extremities, lesions, autoimmune disease, 6p24.1 6p25.3, hair, loss, article, lupus erythematosus, puppies, clinical examination, face, ears, plan, animals, condition, digits, veterinarian, management, diagnosis, immune system, form, differential diagnosis, home, summary, age, welfare, research, examination, mode, fact, laboratory, signs, expressivity, type, diet, skin and muscles, information, dermatopathies