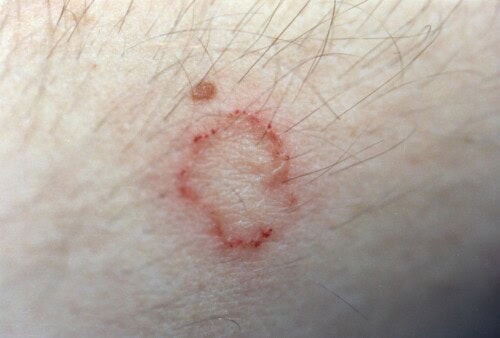
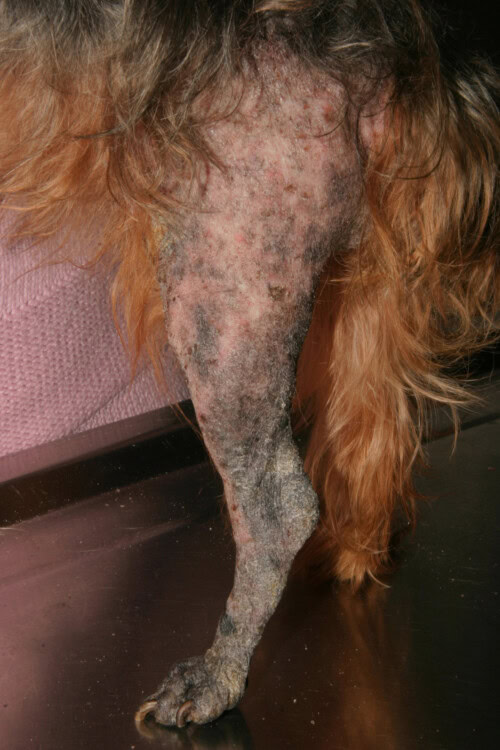
Ringworm, which is a dermatophytosis, represents a superficial fungal infection of the skin and its appendages. This skin condition, caused by keratinophilic fungi belonging to the Microsporum and Trichophyton genera, is of particular importance in veterinary medicine due to its contagious, infectious, and zoonotic potential.
Introduction
Although generally considered a benign condition in immunocompetent dogs, its clinical management remains a challenge due to its polymorphic presentation and its impact on public health.
Etiology and Pathogens
Dermatophytes responsible for infections in dogs are divided into three categories based on their natural habitat:
Zoophilic species, adapted to life on animal hosts, constitute the major pathogens. Microsporum canis represents the predominant etiological agent (40-80% of cases), followed by Trichophyton mentagrophytes (5-35% of cases).
Among geophilic species, Microsporum gypseum accounts for a significant number of cases, up to 25%. These fungi, naturally present in the soil, are associated with the decomposition of keratin from organic debris.
Microsporum persicolor deserves special attention due to its increased prevalence in hunting and working dogs, likely due to more frequent contact with wild rodents and contaminated soil.
Epidemiology and Risk Factors
Global Distribution and Prevalence
Epidemiological analysis of canine dermatophytosis highlights a complex and multifactorial distribution. An in-depth meta-analysis, covering studies conducted in 29 countries, reveals considerable variations depending on geographical contexts and the canine populations studied. In daily veterinary practice, the prevalence of this condition ranges from 0.26 to 5.6% of dermatological cases, thus emphasizing its relatively infrequent nature compared to other dermatoses.
Geographical Distribution and Environmental Influence
Epidemiological data show a particular geographical distribution, with marked prevalence in areas characterized by a warm and humid climate. This trend is particularly observed in Brazil, Chile, India, Italy, and the southern regions of the United States. This specific geographical distribution suggests a close correlation between climatic conditions and the ecology of dermatophytes, influencing their survival and propagation capacity in the environment.
Accurate determination of prevalence and racial predispositions faces several methodological obstacles. Indeed, this dermatophytosis is not a reportable disease, its natural course can spontaneously resolve in many immunocompetent animals, and its clinical presentation varies considerably in severity. These factors potentially introduce bias in the interpretation of epidemiological data.
Analysis of risk factors highlights several key elements:
Age is a predominant factor, with young animals showing increased susceptibility. This vulnerability could be explained by the immaturity of their immune system and the lack of prior exposure to pathogens.
Racial Predispositions and Comparative Analysis
Epidemiological studies highlight significant variations in susceptibility among canine breeds. Yorkshire Terriers show a particularly marked predisposition to dermatophyte infections, especially M. canis. This vulnerability is expressed in both superficial and nodular forms of the infection. Analysis of clinical data corroborates this observation: a study of 55 cases of dermatophytosis revealed that 23.6% of affected dogs belonged to this breed. This overrepresentation was confirmed by a second study where Yorkshire Terriers accounted for 37% of cases (10 out of 27 dogs). This predisposition could be explained by genetic factors influencing the immune response or the composition of the skin barrier, although the precise mechanisms remain to be elucidated.
Working and hunting dogs, on the other hand, show a distinct susceptibility profile, characterized by increased vulnerability to M. persicolor and M. gypseum infections. This epidemiological particularity specifically affects breeds such as the German Shorthaired Pointer, Fox Terrier, Labrador Retriever, Belgian Shepherd Groenendael, Beagle, and Jagdterrier. This predisposition seems more related to behavioral and environmental factors than to intrinsic sensitivity, as these breeds are frequently exposed to contaminated soils and wild reservoirs during their activities.
Hunting and working dogs present a distinct risk profile. Breeds such as German Shorthaired Pointers, Fox Terriers, Labrador Retrievers, Belgian Shepherd Groenendaels, and Beagles show increased susceptibility to M. persicolor and M. gypseum infections. This predisposition is likely explained by their frequent contact with contaminated soil and wild animal reservoirs.
Immune status plays a decisive role in susceptibility to dermatophyte infections. Immunosuppressive conditions, particularly hyperadrenocorticism, can promote the development of more severe and prolonged infections. Scientific literature documents cases of dermatophytosis in association with various systemic conditions: leishmaniosis (4 cases), leishmaniosis associated with ehrlichiosis (1 case), and diabetes mellitus (1 case). The coexistence of concomitant demodicosis, although rarely reported in the literature, is probably a more frequent association than suggested by available publications.
Transmission of dermatophytosis occurs mainly through direct contact with an infected animal or through contaminated objects, including grooming equipment, bedding, collars, and ectoparasites. Skin microtrauma is an essential predisposing factor in the establishment of clinical infection. M. canis infections typically result from contact with an infected animal, mainly cats, while pure environmental transmission remains inefficient. Trichophyton infections are generally associated with contact with infected rodents or their nests. M. gypseum infections, less common, are presumed to result from contact with contaminated soil, reflecting the geophilic nature of this organism.
Ringworm in humans
Pathogenesis and Immune Response
The establishment of a dermatophyte infection results from a complex sequence of molecular and cellular events, orchestrated by interactions between the pathogen and the host. This process unfolds in three distinct and sequential phases, each characterized by specific mechanisms.
The initial adhesion phase is a critical step occurring within 2-6 hours after exposure. This adhesion is mediated by specific adhesins expressed on the surface of arthroconidia, as well as by dermatophyte proteases, particularly subtilases. These enzymes secreted by the fungus play a decisive role in establishing the infection, facilitating adhesion to host corneocytes.
The second phase, marked by the germination of fungal conidia, begins between 4 and 6 hours after initial adhesion. During this stage, germ tubes emerge from the arthroconidia and penetrate the stratum corneum. This invasion has been meticulously documented through in vitro models using isolated corneocytes and reconstituted epidermis, allowing observation of infection progression.
The third phase corresponds to the active invasion of keratinized structures. Fungal hyphae colonize the stratum corneum, developing in various directions, with a marked preference for the follicular unit in most veterinary dermatophytes. This colonization is accompanied by intense enzymatic activity, including the secretion of endoproteases (subtilases and fungalysins) and exoproteases that degrade keratin into assimilable peptides and amino acids. A sophisticated mechanism involving a sulfite efflux pump, encoded by the SSU1 gene, facilitates keratin degradation by cleaving disulfide bonds. The regulation of sulfite formation from cysteine depends on a key enzyme, cysteine dioxygenase (Cdo1).
Dermatophytes have developed elaborate strategies to counteract the host’s immune response. These mechanisms include lymphocyte inhibition by cell wall mannans, alteration of macrophage function, and modification of keratinocyte turnover. However, the host deploys a complex immune response involving humoral and cellular components.
Natural infection with M. canis induces immediate and delayed cutaneous responses to fungal proteins, accompanied by elevated antibody titers and altered lymphoblast response to fungal antigens. Immunological studies have revealed that cats infected or previously exposed to M. canis show significantly higher lymphocyte reactivity to dermatophyte antigens compared to uninfected controls. Although lymphocyte reactivity is similar in culture-positive animals and infected/recovered animals, antibody titers are significantly higher in the culture-positive group. This observation suggests that increased lymphocyte reactivity could represent a Th1-mediated cell response, while antibodies would more reflect exposure without conferring significant protection.
Clinical healing and protection against reinfection essentially depend on a robust cell-mediated immune response, involving effector cells like macrophages and neutrophils, orchestrated by various cytokines, particularly interferon-gamma. This adaptive immune response contributes not only to the elimination of active infection but also to the establishment of protective immunity against subsequent infections.
Clinical Presentation and Differential Diagnosis
Canine dermatophytoses exhibit remarkable clinical polymorphism that reflects the pathogenesis of fungal infection and the host’s immune response. Initial manifestations generally appear one to three weeks after exposure to infectious arthrospores. Classic lesions are characterized by well-demarcated, circular alopecic areas with peripheral extension, generally asymmetrical. Erythema, particularly visible on areas of light skin, is accompanied by scales and crusts due to moderate exudation. Pruritus, variable in intensity, generally remains minimal to absent, although its presence can exacerbate lesions by self-trauma.
Ringworm in a Yorkshire Terrier
Atypical clinical presentations deserve special attention:
Pustular dermatophytosis represents a rare but significant form, which can clinically and histologically mimic pemphigus foliaceus. This particular manifestation highlights the importance of including dermatophytosis in the differential diagnosis of pustular dermatoses.
Kerions constitute an inflammatory form characterized by erythematous, alopecic, dome-shaped, and exudative nodules. Histopathological examination reveals granulomatous or pyogranulomatous formation, often associated with hair shaft fragments containing fungal spores. This presentation is observed mainly during M. gypseum or T. mentagrophytes infections.
Pseudomycetomas and mycetomas represent rarer but significant complications. Clinically, they manifest as single or multiple nodules that fistulate and ulcerate, draining sero-purulent material containing tissue grains. Histopathological examination reveals granulomatous dermatitis or panniculitis containing granules formed by hyphae or pseudohyphae of the fungus.
Differential diagnosis requires a systematic approach and must consider:
Bacterial folliculitis and demodicosis are the main differential diagnoses. In the case of bacterial folliculitis, the presence of follicular papules or pustules, epidermal collarettes, and a moth-eaten appearance of the coat are characteristic. Demodicosis is distinguished by the presence of comedones and a clear demarcation of alopecic areas.
Autoimmune diseases, particularly pemphigus foliaceus and cutaneous lupus erythematosus, should be considered, especially for facial lesions. Pemphigus foliaceus is characterized by non-follicular pustules, crusts, and alopecia. Discoid lupus erythematosus frequently produces scaly, papulopustular, or crusting lesions affecting the nasal planum, unlike dermatophytosis.
Alopecia areata and pseudopelade are distinguished by circular areas of alopecia where the skin most often appears normal.
Generalized exfoliative dermatoses include a wide range of conditions: endocrinopathies, leishmaniosis, exfoliative lupus erythematosus, canine sebaceous adenitis and cutaneous lymphoma. These conditions may present clinical similarities to generalized forms of dermatophytosis.
The clinical presentation can be modified by several factors, including the species of dermatophyte involved, the host’s immune status, and prior administration of treatments. M. persicolor infections, for example, are initially characterized by non-follicular involvement with erythema, scales, and crusts, with alopecia appearing only secondarily. This particular form preferentially affects hunting dogs and frequently manifests with facial lesions.
Conclusion
Ringworm represents a complex dermatological condition whose variable clinical presentation requires a rigorous diagnostic approach. Its therapeutic management must take into account not only treatment efficacy but also zoonotic and environmental aspects. A thorough understanding of its pathogenesis and clinical manifestations is essential to optimize its management.
Frequently Asked Questions (FAQ)
-
Does the presence of spores on the coat systematically indicate an active infection? No, the presence of spores may merely reflect mechanical carriage without follicular invasion.
-
Are immunosuppressed dogs at increased risk of severe infection? Yes, particularly in animals with hyperadrenocorticism where the infection can be more severe and prolonged.
-
Does environmental contamination represent a major risk of transmission? No, studies show that transmission by direct contact with an infected animal is much more frequent than environmental contamination alone.
-
Can we rely solely on clinical appearance for diagnosis? No, the polymorphic presentation of dermatophytosis requires diagnostic confirmation by complementary examinations.
Bibliography
-
Begum, J., & Kumar, R. (2020). Prevalence of dermatophytosis in animals and antifungal susceptibility testing of isolated Trichophyton and Microsporum species. Tropical Animal Health and Production, 53(3), 1-8.
-
Moriello, K. A., Coyner, K., Paterson, S., & Mignon, B. (2017). Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Veterinary Dermatology, 28(3), 266-e68.
-
Pin, D. (2017). Non-dermatophyte dermatoses mimicking dermatophytoses in animals. Mycopathologia, 182(1-2), 1-13.
-
Yamada, S., Anzawa, K., & Mochizuki, T. (2019). An epidemiological study of feline and canine dermatophytoses in Japan. Medical Mycology Journal, 60(2), 39-44.
-
ESCCAP. (2016). Control of dermatophytes in dogs and cats – Adaptation of ESCCAP guideline no. 2 for Switzerland, August 2016. (Guideline for the control of dermatophytoses)
Related Searches
dog, ringworm, ringworm in dogs, clinical signs, body, ringworm treatment, trunk, face, hair, mycosis, people, humans, outdoor environment, loss, limbs, patches, modes, environment, symptoms, arms, man, UV lamp, bleach, prevention, ringworm symptoms, horses, itching, guinea pigs, dog ringworm, dog’s ringworm, rabbits, dog ringworm, ringworm, dog, clinical signs, ringworm in dogs, disease, in dogs, animal, treatment, skin, fur, fungi, microsporum canis, UV lamp, causes, symptoms, pet, development, dog ringworm, texts, prevention, owner, humans, practices, health, appearance, mycosis, outdoor environment, veterinarian, loss, dermatophytes, infection, surfaces, trichophyton mentagrophytes, environment, cat, well-being, examination, lesions, bleach, fact, dog has ringworm, dermatophytoses, spores, diagnosis, ringworms, ringworm treatment, keratin, immune system, body, contact, dermatophytosis, contagion, hair loss, terriers, rodents, cases, how to prevent ringworm, contagiousness, ringworm is a disease, lamp, burden, fall, claws, care, breeds, owners, wood, prevent ringworm, skin lesions