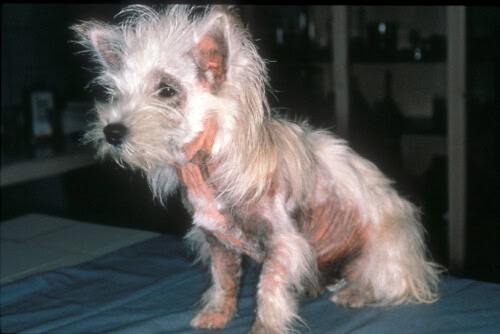
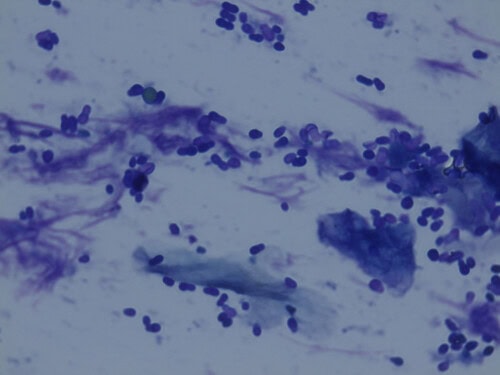
Malassezia is a yeast naturally present on the skin and mucous membranes of dogs and cats. In the event of an underlying disease, it can proliferate and cause skin problems (dermatitis, otitis, paronychia). Diagnosis is simple via cytology, but treatment requires a comprehensive approach, including identification and treatment of the underlying cause.
Introduction
Malassezia is a genus of commensal yeast on the skin and mucous membranes of dogs and cats. They can proliferate in the presence of an underlying disease, causing dermatitis, otitis externa, or secondary paronychia. As allergic dermatitis is one of the most common underlying causes, diagnostic investigation is often indicated. Cats can present with various other underlying problems, especially when Malassezia dermatitis is generalized. This article reviews the clinical presentation, underlying comorbidities, diagnostic considerations, and treatment of Malassezia dermatitis in dogs and cats, in light of emerging evidence of antifungal resistance.
Physiopathological aspects of Malassezia-associated disease
Malassezia metabolism
All Malassezia species, including M. pachydermatis, are lipophilic and require an exogenous lipid source for their growth. The main lipid sources on the skin are triglycerides and free fatty acids produced by the sebaceous glands. Cholesterol and cholesterol esters are produced during natural cell turnover and keratinocyte degeneration. These skin lipids positively influence yeast growth.
Malassezia can also assimilate phospholipids and use organic and inorganic nitrogen. Nitrogen sources can lead to the formation of indole alkaloids, which serve as ligands to the aryl hydrocarbon receptor (AhR) in humans, thus influencing the function of various cells expressing this receptor and modulating different biological responses associated with inflammation, immune homeostasis, skin pathology, skin microflora, and carcinogenesis. In vitro growth of Malassezia can be stimulated by the addition of asparagine, pyridoxine, or thiamine. Malassezia cannot metabolize sugars, but M. pachydermatis is capable of assimilating certain carbohydrates such as mannitol, glycerol, and sorbitol.
Malassezia adhesion
The interaction of Malassezia with keratinocytes is the fundamental starting point for yeast invasion of the host. In vitro adhesion of M. furfur and M. pachydermatis to keratinocytes peaks at approximately 2 hours. Trypsin-sensitive cell surface proteins/glycoproteins also play a role in canine keratinocyte adhesion. Furthermore, an in vitro study showed that Malassezia uses glycosaminoglycans (GAGs) as surface receptors to bind to keratinocytes, but not to dermal fibroblasts.
How Malassezia interacts with melanocytes is not completely understood, although its metabolites, notably azelaic acid, lipoperoxides, and malassezin, have been shown to play a significant role due to their cytotoxic and apoptotic potential. This could explain the depigmented to hypopigmented type of human pityriasis versicolor. However, the number of melanocytes does not appear to be affected in diseased skin; rather, it is the amount of melanin in melanosomes and the distribution of melanosomes in the skin that differ.
Immune response to Malassezia
Malassezia can proliferate in the stratum corneum, the outermost layer of the epidermis, leading to the accumulation of various metabolites and allergens. Epidermal Langerhans cells and keratinocytes recognize Malassezia antigens, leading to the formation of specific antimicrobial peptides and cytokines, resulting in either a “neutral” commensal state (Il-10, TGF-β) or an inflammatory response (Il-1, Il-6, Il-8, TNFα). In addition, epidermal Langerhans cells (and dermal dendritic cells if antigens penetrate through the epidermis into the dermis) migrate to regional lymph nodes, stimulating naive T-helper lymphocytes to proliferate and differentiate into Th1 and/or Th2 cells depending on the cytokine profile (Il-12, Il-4, Il-13). Activated B lymphocytes differentiate into plasma cells and produce different antibodies (IgG or IgE) depending on their co-stimulatory factors. Il-2 and IFNγ induce IgG, while Il-4 and Il-13 lead to an IgE antibody response. IgG can be protective, while IgE can lead to sensitization and mast cell activation.
Malassezia species
M. pachydermatis is the predominant species on healthy dog and cat skin, although it is comparatively less frequently isolated in cats. Other species reported in dogs include M. sympodialis, M. furfur, M. obtusa, M. restricta, M. arunalokei, M. nana, M. slooffiae, and M. globosa. In cats, M. furfur, M. yamatoensis, M. japonica, M. dermatis, M. nana, M. obtusa, M. slooffiae, M. sympodialis, and M. restricta have been reported. Overall, these other species tend to be more common in cats than in dogs.
Clinical presentation of Malassezia dermatitis
Breed predisposition
Malassezia dermatitis is common in dogs, but rare in cats. There is no clear sex or age predisposition. In dogs, West Highland White Terriers, American Cocker Spaniels, Dachshunds, Boxers, Poodles, English Setters, Australian Silky Terriers, Shih Tzus, and Basset Hounds are breeds with an increased risk of Malassezia dermatitis. In cats, Devon Rex and Sphynx cats have a high load of colonizing Malassezia, predisposing them to oily seborrhea and Malassezia-related skin diseases.
Underlying diseases
Malassezia typically causes superficial dermatitis, otitis externa, and/or paronychia. In dogs, this occurs as a result of ectoparasites, allergies (flea bite hypersensitivity, food and environmental atopic dermatitis), endocrinopathies (hyperadrenocorticism, hypothyroidism, diabetes mellitus), superficial pyoderma, keratinization disorders, or, rarely, autoimmune diseases. All these diseases alter the yeast environment favorably. In cats, allergic dermatitis, feline leukemia virus (FeLV) infection, feline immunodeficiency virus (FIV) infection, thymoma-associated exfoliative dermatitis, superficial necrolytic dermatitis, and paraneoplastic alopecia are reported triggers of generalized Malassezia dermatitis. Other predisposing factors such as genetics, ambient humidity, anatomical conformation (skin fold formation), and drug administration (e.g., immunomodulatory drugs) also contribute to the development of Malassezia dermatitis in cats and dogs.
Affected body areas and clinical presentation
Malassezia dermatitis can be localized or generalized. In dogs, commonly affected areas include the ear pinnae, external ear canals, muzzle, ventral neck, ventral body sites, medial thighs, and paws. In most cats, the ear pinnae, face, chin, neck, limbs, and ventral abdomen are most frequently affected, while in Sphynx and Devon Rex breeds, the ventral neck, armpits, inguinal areas, and paws are primarily affected. Additionally, claw folds (in cats) and skin folds (e.g., in brachycephalic breeds) can be affected.
Pruritus is variable. Skin lesions include alopecia, erythema, crusts, scales, lichenification, and accumulation of keratosebaceous debris, while ears or nails often present with a brown to black, foul-smelling exudate. Malassezia dermatitis is usually triggered by a primary underlying disease. A definitive diagnosis is easily obtained by direct cytology and microscopy.
Diagnosis and identification of Malassezia
Direct evaluation and interpretation
A useful, practical, and rapid method for diagnosing Malassezia dermatitis is direct microscopic examination of tape strips, impression smears, cotton swabs, or skin scrapings. For better visualization of fungal elements, potassium hydroxide or special stains such as lactophenol blue, methylene blue, Gram stain, or fluorescent stains can be added to the clinical sample. Malassezia is recognizable by its typical unipolar budding, often broad-based, referred to as “footprint,” “babushka,” or “peanut.”
Due to many influencing factors, there is no clear threshold for the number of Malassezia organisms considered pathological in cytology. Some researchers suggest that more than two organisms per high-power field (hpf) may be considered abnormal. An increased number of Malassezia in the absence of inflammatory cells is defined as “Malassezia overgrowth,” while the presence of inflammatory cells indicates “Malassezia dermatitis.” The authors suggest not interpreting the number of organisms detected by cytology alone, but considering it in conjunction with the history and clinical presentation. For example, two Malassezia organisms per hpf found in a patient with chronic dry dermatitis with abundant keratosebaceous debris and a rancid odor might be more relevant than a higher number of Malassezia organisms in a relatively normal-appearing skin area. Any presence of clinical lesions or pruritus associated with a low number of Malassezia in cytology should be an indication for topical antiseptic treatment to assess how much the yeasts are contributing to the present clinical signs.
Culture isolation
The contact plate technique, where an agar surface is pressed against the test surface, has been used in human and veterinary medicine; it is practical, easy to perform, quick, and inexpensive. Malassezia colonies can be grown on different growth media. Modified Dixon agar or Sabouraud dextrose agar (SDA), commonly used, can be analyzed after 3-7 days of incubation at 32-34 °C. The percentage of carbon dioxide can affect the isolation frequency and colony count when SDA is used. Modified Dixon agar is the preferred medium for Malassezia, as lipid supplementation makes it suitable for lipophilic yeasts when species other than M. pachydermatis are involved. Other culture media such as Leeming-Notman, Ushijima, or Candida chromogenic agar, all supplemented with lipid components, are also suitable.
Other Malassezia culture techniques involve collecting skin scrapings, dry or wet swabs, and adhesive strips. In a canine study comparing adhesive strip sampling to dry swab sampling in dogs with chronic dermatitis, the adhesive strip proved to be the best method.
Skin biopsies and histopathology
As Malassezia can be easily detected in cytological samples, skin biopsies are generally not performed to confirm a diagnosis of Malassezia dermatitis. In histological preparations of skin biopsies from affected patients, Malassezia may be absent due to skin preparation and/or sample processing, or changes may be non-specific. Histological changes associated with Malassezia dermatitis include pronounced irregular epidermal and infundibular hyperplasia, prominent parakeratotic hyperkeratosis, diffuse intercellular edema, and lymphocytic exocytosis, eosinophilic pustulosis, and perivascular to interstitial superficial dermatitis with lymphocytic predominance. If present, unipolar budding yeasts may be found on the surface or in the infundibular keratin.
Molecular biology-based identification methods
For rapid Malassezia detection and species determination, DNA sequence analysis of ribosomal rRNA genes is typically performed using PCR-based methods. These genes are highly conserved among different Malassezia species. Commonly analyzed genes in the rRNA gene cluster include domains 1 (D1) and 2 (D2) of the 26 S subunit, the 18 S small subunit, and the internal transcribed spacer regions (ITS1, ITS2) and 5.8 S. Other useful secondary genes are chitin synthase 2 (CHS2), RNA polymerase 1 (RPB1), RNA polymerase 2 (RPB2), β-tubulin, translation elongation factor 1 alpha (EF1-alpha), mitochondrial cytochrome B (CYTB), and minichromosome maintenance complex component 7 (MCM7).
Treatment
General considerations for Malassezia dermatitis
The underlying primary cause must be identified and corrected, if possible. Depending on the severity and distribution of skin lesions, topical treatment alone may be sufficient, or a combination of topical and systemic treatment may be necessary.
Antifungal sensitivity testing
Despite the regular use of oral antifungal drugs in general practice, antifungal sensitivity testing of Malassezia isolates is rarely performed in veterinary practice, and there is no standardization regarding the method, culture medium, inoculum size, incubation time, and endpoint criteria of minimum inhibitory concentrations (MICs), making data interpretation and recommendations difficult.
An Italian research group evaluated the antifungal susceptibility of M. pachydermatis in various media and concluded that growth in Sabouraud dextrose broth with 1% Tween 80, a stock inoculum suspension of 1-5 × 10^6 CFU/ml, and an incubation time of 48 hours are optimal. Another method using Christensen’s broth with 0.1% Tween 80 and 0.5% Tween 40, an inoculum size of approximately 1-5 × 10^5 yeast cells/ml, and an incubation time of 48 hours can be considered an appropriate alternative.
Standardized international guidelines for Malassezia susceptibility testing are urgently needed so that antifungal susceptibility testing can be widely offered to practicing veterinarians. This would facilitate the detection of Malassezia isolates with increased antifungal minimum inhibitory concentrations and allow for the selection of appropriate antifungal drugs for treatment.
Antifungal susceptibility
M. pachydermatis, the primary species responsible for Malassezia dermatitis and/or otitis externa in small animals, can show resistance to azoles, as demonstrated by two studies using clinical isolates. Reduced azole susceptibility in Malassezia can be caused by missense mutations, amino acid alterations, quadruple tandem duplication, increased ERG11 expression, efflux pump alterations caused by overexpression of genes encoding for ABC transporters (CDR1/CDR2) or major facilitator superfamily (MDR1) membrane transporters, as well as biofilm formation. An East Asian study of atopic dogs, using Etest (Sabouraud dextrose agar with 0.5% Tween 40), showed higher MIC values for ketoconazole and itraconazole, while in another study in Italy, implementing CLSI reference broth microdilution, clinical isolates from sick dogs showed intermediate to high MIC values for fluconazole, ketoconazole, itraconazole, voriconazole, miconazole, and posaconazole, demonstrating that cross-resistance to multiple azole antifungals can occur. A report using protocol M27-A3 indicated reduced susceptibility (elevated MICs) to amphotericin B, clotrimazole, miconazole, fluconazole, and nystatin, associated with M. pachydermatis strains from sick dogs.
There are a few reports in dogs where treatment failure due to resistance was clinically suspected and later confirmed in vitro by antifungal sensitivity testing. Resistance has been encountered to commonly used systemic and topical agents, including itraconazole, ketoconazole, and clotrimazole (several-fold increase in MIC).
Conclusions
Malassezia dermatitis is common in dogs, but less common in cats. In both species, there are breed predispositions. M. pachydermatis is most commonly isolated in sick dogs and cats. Skin lesions include alopecia, erythema, crusting, scaling, lichenification, and accumulation of keratosebaceous debris, while ears or nails often present with a brown to black, foul-smelling exudate. Pruritus is variable. Malassezia dermatitis is usually triggered by a primary underlying disease. A definitive diagnosis is easily obtained by direct cytology and microscopy. There is a need for standardized and commercially available culture and sensitivity methodology to inform treatment decisions in refractory disease. In patients with Malassezia dermatitis, the focus should be on topical treatment and identification of the underlying disease. Systemic antifungal treatment should be reserved for severe or refractory disease only, due to the risk of azole resistance.
Food For Thought
- Malassezia dermatitis is a common skin condition in dogs, but rare in cats.
- It is often secondary to an underlying disease, such as allergy or endocrinopathy.
- Diagnosis is made by cytology and identification of Malassezia yeast.
- Treatment relies on topical and/or systemic antifungals, as well as management of the underlying disease.
- Antifungal resistance is an emerging problem that requires careful attention.
References
Malassezia dermatitis in dogs and cats. Vet J. 2024 Apr:304:106084. doi: 10.1016/j.tvjl.2024.106084
Related Searches
cat dermatitis, atopic dermatitis, dermatitis, cat, in cats, dermatitis in cats, skin, dermatosis, immune system, dermatitis in, origin, reaction, substances, allergies, veterinary, causes, conditions, all, environment, treatment, disease, need, symptoms, diet, blood test, problems, miliary dermatitis, dairy products, coat, fleas, animal, eczema, reading, itching, pet, basket, element, inflammation, care, patches, case, article, tips, hypersensitivity, management, fatty acids, diagnosis, dog, form, ears, bites, dapp, allergens, lesions, way, companion, life, food supplements, mites, manifestations, allergic rhinitis, hair, parasites, superinfections, clinical signs, disorders, flea bites, allergic dermatitis, medications, atopic eczema