The spectacular demographic growth of the French Bulldog over the past decade has, through a genetic bottleneck effect and extreme phenotypic selection, exacerbated the prevalence of complex dermatological disorders. Overview of the main dermatoses affecting them and their management.
Anatomical and genetic particularities of the French Bulldog
Prevalence of atopic dermatitis and breed vulnerability
The French Bulldog occupies a unique position in veterinary dermatology, not only due to its growing popularity in Western countries, but especially because of the unique convergence of anatomical, genetic and immunological factors that predispose this breed to an exceptionally wide dermatological pathological spectrum. Recent epidemiological data reveal an atopic dermatitis prevalence reaching 15 to 20% in this canine population, a rate three times higher than the inter-breed average. This cutaneous vulnerability results from extreme brachycephalic body architecture associated with documented genetic anomalies affecting epidermal cohesion and immune response.
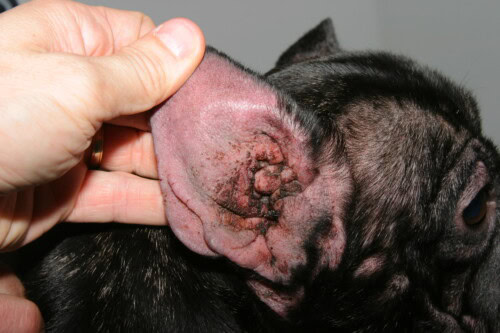
Impact of brachycephalic conformation on intertrigo
The brachycephalic conformation of the French Bulldog generates major dermatological repercussions through the creation of multiple cutaneous fold zones. The cranio-facial shortening, resulting partly from a mutation of the BMP3 gene, engenders excess facial cutaneous tissue that folds into deep wrinkles, notably at the naso-facial region, the inter-labial groove and the peri-vulvar zone in females. These anaerobic microenvironments present relative humidity superior to 80%, elevated temperature of 2 to 3 degrees compared to exposed areas, and permanent occlusion that favors microbial proliferation. The pathophysiology of resulting intertrigo is based on initial epidermal maceration followed by cutaneous barrier rupture, allowing colonization by Staphylococcus pseudintermedius and yeasts of the genus Malassezia. The cutaneous pH in these confined zones progressively alkalinizes, passing from 5.5 to 7.2, creating a favorable terrain for logarithmic bacterial proliferation.
Cytokine receptor overexpression and JAK-STAT pathway
The cutaneous immune system of the French Bulldog also presents documented functional particularities. Transcriptomic analysis of non-lesional cutaneous biopsies in atopic individuals of this breed reveals constitutive overexpression of genes coding for interleukin-31 receptors (IL-31RA) and Oncostatin M Receptor (OSMR), with mRNA levels superior to healthy dogs. This increased expression predisposes to exaggerated activation of the JAK1/JAK2-STAT3 signaling pathway in the presence of even physiological concentrations of IL-31, a major pruritogenic cytokine produced by T helper type 2 lymphocytes. The heterodimeric IL-31RA/OSMR complex phosphorylates JAK1 and JAK2 tyrosine kinases, which then activate STAT3 by phosphorylation of its tyrosine 705, triggering the transcriptional cascade of pruritus. This molecular hypersensitivity explains the intensity of pruritus observed clinically in this breed, even during minimal antigenic stimulations.
Follicular density and sebum deficiency
The follicular density constitutes another anatomical characteristic influencing the dermatological pathology of the French Bulldog. Quantitative histomorphometric studies reveal a lower average follicular density than in long-haired breeds like the Poodle or Yorkshire. This follicular rarefaction is accompanied by proportional reduction in sebaceous secretion, with annexed sebaceous glands being less voluminous. Canine sebum, rich in triglycerides and free fatty acids, actively participates in cutaneous antimicrobial defense through its lipophilic effect on pathogens and its role in maintaining acidic surface pH. The relative sebum deficiency in the French Bulldog alters this first line of defense and favors colonization by opportunistic microorganisms.
Ultrastructural and lipidomic anomalies of the stratum corneum
The stratum corneum architecture also presents ultrastructural anomalies in atopic French Bulldogs compared to healthy individuals of the same breed. Transmission electron microscopy studies reveal enlarged intercorneocyte spacing with lipid lacunae within extracellular lamellae. Lipidomic analysis by liquid chromatography coupled to mass spectrometry demonstrates a 35 to 40% reduction in very long chain ceramides, notably [NP] and [EOS] class ceramides, essential for laminar organization of the intercorneocyte cement. [EOS] ceramides, characterized by sphingosine esterified to linoleic acid, play a critical structural role in anchoring lipid lamellae to the protein cornified envelope. Their deficit compromises the architectural organization of the stratum corneum, increasing permeability to environmental allergens and microorganisms.
Alteration of epidermal renewal and PROM1 gene
The genetic predisposition to atopy in the French Bulldog also involves variations of the PROM1 gene, coding for prominin-1, a transmembrane glycoprotein expressed in epidermal stem cells. A particular variant of this gene, identified by next-generation sequencing in 156 atopic French Bulldogs, is associated with altered epidermal renewal with a prolonged keratinocyte cycle duration of 19 days on average versus 14 to 16 days in healthy dogs. This slowed epidermal kinetics disturbs terminal maturation of keratinocytes and synthesis of barrier components, creating a vicious circle where structural dysfunction favors inflammation, which itself worsens disruption of epidermal differentiation.
Antimicrobial peptide deficit and biofilm formation
The susceptibility to recurrent cutaneous infections observed in this breed also finds an explanation in reduced expression of endogenous antimicrobial peptides. Canine beta-defensins, notably cBD1, cBD3 and cBD103, constitute an essential innate defense against cutaneous pathogens. Quantitative RT-PCR analyses of cutaneous biopsies from atopic French Bulldogs demonstrate 60% reduced cBD103 expression compared to healthy controls, with average mRNA levels of 0.4 relative units versus 1.0. This deficient innate immunity facilitates installation of organized bacterial biofilms, particularly Staphylococcus pseudintermedius, whose eradication becomes progressively more difficult due to matrix protection conferred by the biofilm’s three-dimensional structure.
Module 2: Epidermal barrier dysfunction – Molecular mechanisms
“Brick and mortar” architectural model
The epidermal barrier of the French Bulldog presents structural and functional anomalies that constitute the primary pathophysiological substrate of its dermatological vulnerability. This barrier, located in the stratum corneum, is based on an architectural model called “brick and mortar” where corneocytes represent the bricks and intercellular lipid lamellae form the mortar. In French Bulldogs predisposed to atopy, this system undergoes disorganization at several molecular levels, compromising its function as a rampart against allergen penetration, microbial colonization and trans-epidermal water loss.
Central role of filaggrin in epidermal cohesion
Filaggrin (keratin filament aggregation protein) plays a central role in cutaneous barrier formation. This 400 kDa protein, encoded by the FLG gene, undergoes complex proteolytic maturation during terminal keratinocyte differentiation. In the granular layer, profilaggrin is dephosphorylated then cleaved by specific proteases to generate functional filaggrin monomers. These monomers aggregate keratin filaments into dense macrofibrils, contributing to corneocyte flattening and stratum corneum compaction. In atopic French Bulldogs, immunohistochemical analyses reveal reduced filaggrin expression, with marking intensity decreased by 45% compared to age-matched healthy controls.
Natural moisturizing factors (NMF) and water loss
Subsequent filaggrin degradation generates natural moisturizing factors (NMF) essential for maintaining corneal hydration. Peptidase enzymes, notably caspase-14 and bleomycin hydrolases, fragment filaggrin into free amino acids, including histidine, serine and arginine. Histidine is then deaminated by histidase to trans-urocanic acid, which possesses hygroscopic and photoprotective properties. Arginine is converted to citrulline by peptidylarginine deiminases. In the French Bulldog, high-performance liquid chromatography quantification of the stratum corneum demonstrates a 42% reduction in free amino acid levels and 38% decrease in urocanic acid compared to non-predisposed breeds. This NMF deficiency leads to chronic stratum corneum dehydration with measurable increase in insensible water loss, rising from 12-15 g/m²/h in healthy dogs to 28-35 g/m²/h in atopics.
Tight junction alteration
Tight junctions of the granular layer constitute a second level of epidermal barrier, controlling paracellular permeability. These protein complexes, composed mainly of claudins (claudin-1, claudin-4), occludin and zonula occludens proteins (ZO-1, ZO-2), form an anastomosed network creating intercellular sealing. Transcriptomic analyses of atopic French Bulldog skin reveal significant under-expression of CLDN1 and OCLN genes, coding respectively for claudin-1 and occludin, with mRNA expression reductions of 2.1 and 1.7 fold. This tight junction alteration allows facilitated penetration of high molecular weight allergens, normally excluded by this selective barrier, triggering inappropriate activation of the cutaneous immune system.
Disruption of epidermal lipid metabolism
Epidermal lipid metabolism presents major quantitative and qualitative anomalies in this breed. Stratum corneum lipids, synthesized in granular layer keratinocytes and stored in Odland-type lamellar bodies, are secreted by exocytosis into the intercorneocyte space. These lipids comprise three main classes: ceramides (50%), cholesterol (25%) and free fatty acids (10-15%), in an optimal 1:1:1 molar ratio necessary for formation of impermeable orthorhombic lamellae. In atopic French Bulldogs, gas chromatography coupled to mass spectrometry analyses demonstrate lipid ratio disruption, with relative ceramide reduction to 38% and compensatory cholesterol increase to 35%, altering lamellar crystallinity and increasing permeability.
Importance of [EOS] class ceramides
Ceramides, complex sphingolipids composed of sphingosine linked to a fatty acid by an amide bond, are subdivided into 12 classes according to their sphingoid base and fatty acid nature. [EOS] class ceramides (acylceramides containing linoleate esterification) are particularly critical for cutaneous impermeability. Their biosynthesis involves an enzymatic cascade comprising serine palmitoyltransferase, ceramide synthases (notably CerS3 for ultra-long fatty acids), and glucosylceramide-beta-glucosidase which hydrolyzes glucosylceramides to free ceramides. Western blot studies on epidermal protein extracts from atopic French Bulldogs reveal 52% reduced CerS3 expression compared to controls, correlating with decreased very long chain ceramides (C28-C32).
Beta-glucocerebrosidase (GBA) activity
The beta-glucocerebrosidase (GBA) enzyme, located in lamellar bodies, catalyzes the terminal conversion step of glucosylceramides to functional ceramides at secretion into extracellular space. Deficiency in this enzyme, documented in certain atopic dogs, leads to accumulation of non-functional glucosylated precursors and deficit in mature ceramides. GBA enzymatic activity, measured by fluorometry on stratum corneum homogenates from French Bulldogs, proves reduced by 38% on average in atopic subjects, suggesting disruption of the post-secretory lipid maturation process.
Role of sphingomyelin deacylase (SMase)
Sphingomyelin deacylase (SMase), also known as alkaline ceramidase, regulates ceramide homeostasis by catalyzing their hydrolysis. A delicate balance between ceramide synthesis and degradation determines their tissue concentration. In the French Bulldog, enzymatic studies suggest relative hyperactivity of certain ceramidases, contributing to observed ceramide depletion. Gene expression of ASAH2, coding for neutral ceramidase, is increased 1.6-fold in atopic lesional skin, favoring excessive ceramide catabolism that worsens barrier dysfunction.
Free fatty acid and linoleic acid deficit
Free fatty acids of the stratum corneum, mainly linoleic acid (C18:2 ω-6), oleic acid (C18:1 ω-9) and palmitic acid (C16:0), actively participate in lamellar organization and possess intrinsic anti-inflammatory properties. Linoleic acid, an essential fatty acid, incorporates into [EOS] ceramides via esterification of the ω-hydroxycéramide residue. Its dietary or metabolic deficiency leads to its replacement by oleic acid, creating structurally abnormal [EOO] ceramides that disrupt lamellar organization. Comparative lipidomic analyses reveal that 23% of atopic French Bulldogs present a linoleic/oleic ratio below 2.5 in the stratum corneum, a threshold below which barrier function significantly deteriorates.
Calpain-1 hyperactivation and desmosomal degradation
Calpain-1, a calcium-dependent protease, regulates controlled proteolysis of desmoglein-1 and corneodesmosin, intercorneocyte adhesion proteins. This physiological proteolysis allows orderly desquamation of the corneal layer. However, in an inflammatory context, calpain-1 hyperactivation by Th2 cytokines, notably IL-4 and IL-13, accelerates premature desmosomal degradation, creating micro-fissures in the stratum corneum. In atopic French Bulldogs, calpain-1 enzymatic activity, measured by fluorogenic substrates, proves increased 2.8-fold in lesional skin, correlating with pathological desquamation and worsened permeability.
Cornified envelope fragility
The cornified envelope, a highly cross-linked protein structure formed under the corneocyte plasma membrane, constitutes an essential architectural skeleton. Its formation involves epidermal transglutaminase-1 which catalyzes isopeptide bond formation between precursor proteins (loricrin, involucrin, small proline-rich proteins). Loricrin represents 70 to 80% of envelope protein mass. Biochemical analyses of isolated corneocytes from atopic French Bulldogs reveal deficient envelope cross-linking, with mechanical resistance reduced by 35% measured by micromechanical traction tests. This structural fragility favors premature corneocyte fragmentation and breach formation in the barrier.
Module 3: Atopic dermatitis – Epidemiology and racial pathogenesis
Early atopic dermatitis in a French Bulldog
General characteristics of canine atopic dermatitis
Canine atopic dermatitis represents the most frequent dermatological pathology in the French Bulldog, affecting between 15 and 20% of the breed population according to European and North American epidemiological studies. However, this should be nuanced with certain recruitment biases. This chronic recurrent inflammatory dermatosis is characterized by intense pruritus, stereotyped lesional distribution and clinical evolution marked by flares interspersed with partial remissions. The complex pathogenesis of this condition involves interaction between polygenic genetic predisposition, primary epidermal barrier dysfunction, immune dysregulation with excessive Th2 polarization, and triggering environmental factors including airborne and food allergens.
Chronology and precocity of clinical signs
The age of onset of clinical manifestations in the French Bulldog typically occurs between 6 months and 3 years, with an incidence peak between 12 and 18 months. A retrospective study of 487 atopic French Bulldogs demonstrates that 62% of cases manifest before 24 months of age, and 89% before 4 years. This early onset contrasts with certain breeds like the German Shepherd where signs can debut later. Initial clinical expression is frequently characterized by isolated facial pruritus, affecting the periocular area, ear pavilion and labial commissures, progressively evolving toward generalization with pedal, axillary and ventral involvement over 6 to 18 months. The CADESI-4 score (Canine Atopic Dermatitis Extent and Severity Index), evaluating erythema and lesions on 20 anatomical sites, reveals average values of 35 to 45 in untreated French Bulldogs, compared to 25-30 for other atopic breeds, testifying to increased clinical severity.
Atopic otitis
Lesional distribution particularities and hypermastocytosis
Lesional distribution in this breed presents certain particularities compared to the classic pattern of atopic dermatitis. While involvement of classic areas (face, ears, interdigital spaces, axial and inguinal folds) remains constant, the French Bulldog frequently develops severe lesions at the level of facial folds and naso-labial groove, where maceration worsens underlying atopic inflammation.
The Major Discovery of the SLAMF1 Variant
The recent discovery of a major genetic variant associated with canine atopic dermatitis constitutes a considerable advance in understanding the mechanisms underlying dermatological predispositions of the French Bulldog. A genome-wide association study conducted on more than 28,000 dogs genotyped by medium-density single nucleotide polymorphism arrays identified a statistically significant signal on canine chromosome 38, particularly intense in the French Bulldog. Complete genome sequencing of cases and controls revealed a splice site donor variant in the SLAMF1 gene, coding for signaling lymphocyte activation molecule type 1, also designated CD150. The SLAMF1 mutation alters the highly conserved splice donor site downstream of exon 4, causing aberrant cryptic site use and addition of 41 base pairs to exon 4. This modification generates a truncated protein with 83 aberrant amino acids before early termination, thus suppressing the SLAMF1 cytoplasmic tail including ITSM functional motifs essential for cellular signaling ligand binding.
The prevalence of this variant is particularly alarming in the French Bulldog. Data indicate this variant is present in 71% of breed individuals, versus 40% in Boxers, suggesting involuntary genetic fixation linked to breed selection. The clinical impact is direct and quantifiable: the presence of this variant significantly increases the risk of developing canine atopic dermatitis (CAD). Relative risk analyses show that dogs carrying one copy of the variant have an increased risk of 1.89 times, while homozygotes for the variant see their risk multiplied by 3.57.
The SLAMF1 receptor, expressed on the surface of T, B, NK lymphocytes and dendritic cells, plays a critical regulatory role in immune responses. Cytoplasmic tail disruption prevents SAP adaptor protein binding and inhibits ternary SLAM-SAP-Fyn-SH3 complex formation, a signaling cascade involved in modulating inflammatory cytokine production by monocyte-derived dendritic cells. The pathogenic hypothesis rests on inflammatory response dysregulation following allergenic stimuli, with homotypic SLAM-SLAM interactions normally inhibiting pro-inflammatory cytokine production induced by CD40. The SLAMF1 variant allelic frequency is established at 0.082 in the Dog10k database and proves particularly high or even fixed in certain lines of French Bulldogs, Boxers and Boston Terriers. Inheritance mode analysis and odds ratio calculations demonstrate this variant confers doubled risk of developing atopic dermatitis, with dose-dependent effect suggesting dominant or additive inheritance mode depending on studied breeds.
This molecular confirmation validates clinical observation of severe “breed atopy”. SLAMF1 dysfunction could explain the breed’s tendency to develop disproportionate inflammatory responses to environmental allergens, exceeding the simple framework of Dermatophagoides farinae hypersensitivity to include intrinsic immune dysregulation.
Immunological pathogenesis and Th2 polarization
The immunological pathogenesis of atopic dermatitis in the French Bulldog involves a cascade of cellular and molecular events initiated by transcutaneous penetration of environmental allergens through the deficient epidermal barrier. Epidermal Langerhans cells, antigen-presenting dendritic cells, capture allergens and migrate to draining lymph nodes where they activate naïve T lymphocytes. In a genetically predisposed context, this activation preferentially orients differentiation toward Th2 phenotype, characterized by production of interleukins IL-4, IL-5, IL-13 and IL-31. IL-4 and IL-13 induce B lymphocyte isotype switching toward production of allergen-specific immunoglobulin E (IgE), which fix on high-affinity FcεRI receptors expressed by cutaneous mast cells and basophils.
Mast cell degranulation and IgE hyperproduction
IgE-dependent mast cell degranulation, triggered by allergenic re-exposure and membrane IgE crosslinking, releases a plethora of preformed and newly formed mediators. Preformed mediators include histamine, tryptase, chymase and heparin, responsible for vasodilatation, erythema and acute pruritus. Newly formed mediators, synthesized after cellular activation, comprise leukotrienes (LTB4, LTC4) from arachidonic acid metabolism by 5-lipoxygenase, and prostaglandins (PGD2) generated via cyclooxygenase-2.
Central role of interleukin-31 (IL-31) in pruritus
Interleukin-31, a major pruritogenic cytokine produced mainly by activated Th2 lymphocytes, plays a central role in atopic pruritus pathophysiology. This cytokine binds to a heterodimeric receptor complex composed of IL-31RA and OSMR, constitutively expressed on cutaneous sensory neurons and keratinocytes. Activation of this complex triggers sequential phosphorylation of JAK1, JAK2 kinases and STAT1, STAT3 and STAT5 transcription factors, inducing expression of pro-pruritic and pro-inflammatory genes. Serum IL-31 assays in atopic French Bulldogs reveal average concentrations of 485 pg/ml, significantly superior to 125 pg/ml measured in healthy dogs, with positive correlation (r=0.72, p<0.001) between IL-31 levels and pruritus intensity evaluated by visual analog scale.
Response amplification by TSLP
Thymic stromal lymphopoietin (TSLP), an epithelial cytokine produced by keratinocytes in response to mechanical aggression and allergenic proteases, amplifies Th2 response by activating dendritic cells toward pro-allergic phenotype. TSLP induces OX40-ligand expression on dendritic cells, a co-stimulatory molecule essential for Th2 differentiation. Transcriptomic analyses of lesional skin from atopic French Bulldogs reveal 4.2-fold TSLP overexpression compared to healthy skin, with mRNA levels correlating with epidermal thickness and acanthosis degree. This keratinocyte-dendrocyte-T lymphocyte amplification loop perpetuates chronic inflammation and progressive allergenic sensitization.
Recruitment and impact of tissue eosinophils
Eosinophils, major effector granulocytes of type 2 inflammation, accumulate in the dermis of atopic lesions under chemotactic influence of IL-5, eotaxin (CCL11) and RANTES (CCL5). These cells release cytotoxic granular cationic proteins, notably major basic protein (MBP), eosinophil peroxidase (EPO) and eosinophil cationic protein (ECP), which damage surrounding tissues and amplify inflammation. Histological eosinophil counts on biopsies from atopic French Bulldogs show average densities of 215 cells per square millimeter, versus 15 to 25 in healthy controls. This tissue eosinophilia correlates with disease chronicity and presence of lichenified lesions.
Cutaneous microbiome dysbiosis
The cutaneous microbiome of atopic French Bulldogs presents profound alterations in its composition and diversity. 16S rRNA sequencing analyses reveal dysbiosis characterized by alpha diversity loss (Shannon index reduced from 4.2 to 2.1) and clonal expansion of Staphylococcus pseudintermedius, whose relative abundance passes from 5-10% in healthy dogs to 45-65% in lesional zones. This staphylococcal proliferation does not represent simple opportunistic colonization but actively participates in pathogenesis by production of exotoxins with superantigenic activity. Staphylococcal enterotoxins (SEA, SEB, SEC) activate T lymphocytes polyclonally independently of their antigenic specificity, by bridging the T cell receptor (TCR) to the major histocompatibility complex class II (MHC-II), massively amplifying inflammatory response.
Allergenic sensitization profile and polysensitization
Allergenic sensitization in atopic French Bulldogs follows a characteristic profile identified by intradermal tests and specific IgE assays. A European multicenter study of 842 atopic French Bulldogs reveals that 78% present positive reactions to house dust mites (Dermatophagoides farinae, Dermatophagoides pteronyssinus), 56% to grass pollens, 48% to indoor molds (Malassezia sympodialis, Aspergillus fumigatus) and 34% to epithelial allergens. Polysensitization, defined by at least 5 significant reactivities, concerns 67% of individuals, suggesting global allergenic hyperreactivity rather than monoallergenic sensitization. This polysensitization complicates therapeutic management and limits allergenic-specific immunotherapy efficacy.
Module 4: Fold dermatoses and infectious complications
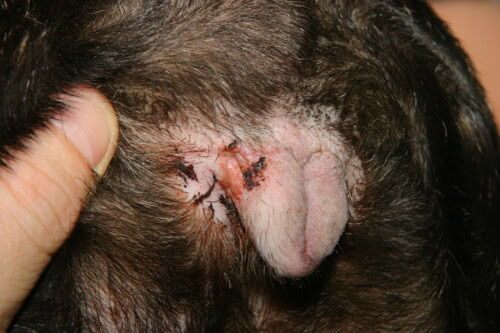
Vulvar intertrigo
Intertrigo pathophysiology
Intertrigo or fold dermatitis constitutes a clinical entity quasi-pathognomonic of the French Bulldog, resulting directly from its extreme brachycephalic conformation. This inflammatory dermatosis develops in cutaneous fold zones where permanent apposition of two epidermal surfaces creates an occluded, hot and humid microenvironment, conducive to tissue maceration and microbial proliferation. Privileged anatomical localizations include the naso-facial fold, lower lip fold, vulvar folds in females, and secondarily tail folds when curled in corkscrew fashion. Intertrigo pathophysiology is based on a cascade of events initiated by continuous mechanical friction between opposed cutaneous surfaces, generating contact inflammation with pro-inflammatory cytokine release by traumatized keratinocytes.
Consequences of maceration and pH alkalization
Epidermal maceration, central process of intertrigo pathogenesis, results from accumulation of lacrimal, salivary and sebaceous secretions in the confined fold space. Relative humidity in these zones reaches 85 to 95%, favoring excessive stratum corneum hydration which loses its structural cohesion. Cutaneous pH measurements by surface electrode reveal progressive microenvironment alkalization in folds, with values passing from 5.5 in exposed cutaneous zones to 7.0-7.5 in deep folds. This pH elevation partially inactivates stratum corneum proteolytic enzymes, normally active in acidic medium, disrupting physiological desquamation and favoring cellular debris accumulation. Alkalization also modifies surface lipid film composition, reducing its natural antimicrobial activity and creating favorable terrain for bacterial and fungal colonization.
Microbial colonization sequence
Microbial colonization of macerated folds follows a predictable sequence, beginning with resident commensal flora expansion, mainly Staphylococcus pseudintermedius and Malassezia pachydermatis yeasts, which evolve toward opportunistic pathogenic status. Quantitative microbiological counts by standardized swab technique reveal average bacterial loads of 10^6 to 10^7 colony forming units per square centimeter in inflamed folds, versus 10^3 to 10^4 CFU/cm² on adjacent healthy skin. Staphylococcus pseudintermedius, gram-positive coagulase-producing cocci, secretes an arsenal of enzymes and toxins favoring tissue invasion, notably lipases, proteases, nucleases and staphylococcal protein A which inhibits opsonization by binding to immunoglobulin Fc fragment. The transition from commensal to pathogen is accompanied by bacterial genomic profile modification with virulence factor overexpression regulated by the agr (accessory gene regulator) quorum sensing system.
Classification and diagnosis of superficial pyodermas
Superficial pyodermas represent the natural evolution of untreated or chronic intertrigo in the French Bulldog. These bacterial cutaneous infections are characterized by neutrophilic infiltration of superficial dermis with intra-epidermal or subcorneal pustule formation. Anatomical classification distinguishes impetigo (superficial epidermal layer involvement), superficial folliculitis (upper follicle third infection) and diffuse superficial pyoderma. In the French Bulldog, superficial bacterial folliculitis manifests clinically as erythematous papules centered with pustules, evolving into epidermal collarettes after pustular rupture. Glabrous or sparsely haired zones like axillary and inguinal regions are preferentially affected. Cytological examination by slide apposition reveals degenerate neutrophilic population with phagocytosis of cocci arranged in clusters (staphylococci) in 92% of cases, confirming bacterial infection origin.
Resistant staphylococci problem (MRSP)
Antibiotic resistance of Staphylococcus pseudintermedius constitutes a growing problem in French Bulldog veterinary dermatology, a breed frequently receiving iterative antibiotherapy for recurrent cutaneous infections. Methicillin-resistant strains (MRSP – Methicillin-Resistant Staphylococcus pseudintermedius) have emerged since the 2000s, acquiring the mecA gene coding for modified penicillin-binding protein (PBP2a) with low affinity for beta-lactams. A prevalence study conducted in 28 French veterinary clinics reveals an MRSP carriage rate of 18% in French Bulldogs consulting for dermatitis, versus 8% average across all breeds. These MRSP strains frequently present multiresistance, associating fluoroquinolone resistance (mutations in gyrA and parC genes coding for DNA gyrase and topoisomerase IV), macrolides (ermB gene acquisition) and aminoglycosides, drastically limiting available therapeutic arsenal.
Persistence by bacterial biofilms
Bacterial biofilms, complex three-dimensional structures where bacteria organize into communities encapsulated in extracellular matrix of polysaccharides, proteins and extracellular DNA, represent a major persistence mechanism of chronic cutaneous infections. Staphylococcus pseudintermedius produces polysaccharide intercellular adhesin (PIA) coded by the icaADBC operon, allowing bacterial adhesion to corneocytes and mature biofilm architecture formation. Sessile biofilm bacteria present increased antimicrobial tolerance of 100 to 1000 times compared to free planktonic forms, resulting from several mechanisms: limited antibiotic penetration through polymeric matrix, unfavorable nutritional microenvironment inducing bacterial metabolic slowdown, and biofilm-specific resistance gene expression. Confocal microscopy of cutaneous biopsies from French Bulldogs with chronic recurrent pyoderma reveals organized biofilm presence in 68% of cases, explaining eradication difficulty by conventional antibiotherapy.
Pyotraumatic dermatitis (hot spot)
Pyotraumatic dermatitis, commonly called hot spot or acute moist dermatitis, constitutes a frequent complication in atopic French Bulldogs. This erythematous exudative lesion with lightning evolution results from intense self-mutilation triggered by localized pruritus, often of allergic or parasitic origin. Compulsive licking and scratching create epidermal erosion rapidly colonized by opportunistic cutaneous flora, mainly Staphylococcus pseudintermedius. Release of inflammatory mediators, notably antimicrobial peptide LL-37 which paradoxically possesses chemotactic properties for neutrophils, massively recruits these granulocytes and amplifies local inflammation. Abundant sero-purulent exudate macerates adjacent hairs, creating a humid malodorous alopecic plaque of 3 to 10 centimeters diameter, typically localized on regions with high follicular density like temporal, pre-auricular or rump regions.
Malassezia yeast complications
Malassezia infections represent a frequent complication of intertrigo and atopic dermatitis in the French Bulldog. These lipophilic commensal yeasts of genus Malassezia, mainly M. pachydermatis (non-lipid-dependent species) and M. sympodialis, proliferate opportunistically in hot, humid and lipidic environments of cutaneous folds. Malassezia dermatitis pathogenesis involves complex interaction between quantitative fungal proliferation and host immunological reactivity. Certain dogs develop type I and IV hypersensitivity to Malassezia antigens, mediated respectively by specific IgE and T lymphocyte cellular response. Cytological counts by Scotch-tape technique reveal yeast densities superior to 10 organisms per high-power microscopic field in affected zones, versus less than 2 organisms in asymptomatic carriers.
External otitis and canal remodeling
External otitis, quasi-constant condition in atopic French Bulldogs, results from cutaneous inflammation extension to external auditory canal, a zone considered as modified cutaneous fold. French Bulldog ear conformation, with short, wide and horizontally oriented external auditory canal, favors cerumen accumulation and humidity retention. Canal epithelium allergic inflammation leads to ceruminous gland hyperplasia with modified cerumen production, more viscous and in excessive quantity, creating optimal nutritive substrate for Malassezia pachydermatis. Cytological analyses of cerumen from French Bulldogs with external otitis reveal Malassezia predominance in 76% of cases, mixed bacterial infection in 18%, and pure bacterial infection in only 6%. Otitic chronicity progressively induces irreversible structural modifications of the canal, with fibrosis, auricular cartilage calcification and progressive stenosis, potentially requiring surgical intervention with canal ablation and bulla osteotomy in terminal stages.
Module 5: Malassezia dermatitis and recurrent pyodermas
Malassezia pachydermatis biology
Malassezia dermatitis constitutes a major clinical entity in the French Bulldog, occurring either as complication of underlying atopic dermatitis, or as manifestation of primary hypersensitivity to fungal antigens. Yeasts of genus Malassezia represent ubiquitous lipophilic commensal organisms of canine skin and mucous membranes, with seven species identified in dogs of which Malassezia pachydermatis remains most frequent. This species presents the unique particularity of being non-lipid-dependent for in vitro culture, contrary to other Malassezia species which require medium lipid enrichment. M. pachydermatis cellular morphology is characterized by ovoid to monopolar blastoconidia of 3 to 8 micrometers with unipolar budding, easily identifiable by cytological examination after rapid staining like Diff-Quik or Gram staining where they appear as violet to blue peanut-shaped cocci.
Transition to pathogenicity mechanisms
The transition from commensal to pathogen of Malassezia yeasts involves several interconnected mechanisms. Complete genomic sequencing studies of M. pachydermatis reveal a 7.98 megabase genome coding for enzymatic arsenal comprising secreted lipases, phospholipases and proteases which degrade cutaneous lipids and epidermal structural proteins. Lipases hydrolyze sebum triglycerides into free fatty acids, some like oleic acid possessing irritant properties and further altering barrier function. Phospholipases A2 secreted by Malassezia cleave keratinocyte membrane phospholipids, generating arachidonic acid precursor of pro-inflammatory prostaglandin and leukotriene mediators via cyclooxygenase and lipoxygenase pathways. This pro-inflammatory enzymatic activity explains how even quantitatively moderate colonization can induce clinically significant inflammation in genetically predisposed individuals.
Immune recognition and inflammation
Immune recognition of Malassezia antigens by the cutaneous immune system constitutes a central pathogenic element. Dermal and epidermal dendritic cells express pattern recognition receptors (PRR), notably Toll-like receptors TLR2 and TLR4, which recognize fungal wall components like mannans and beta-glucans. This recognition triggers NF-κB signaling pathway activation with pro-inflammatory cytokine IL-1β, IL-6, IL-8 and TNF-α production by keratinocytes and resident immune cells. In sensitized French Bulldogs, repeated exposure to Malassezia antigens induces Th2 polarization with production of specific IgE which fix on cutaneous mast cell FcεRI receptors. Antigenic re-exposure then provokes rapid mast cell degranulation with histamine release, triggering pruritus and erythema within minutes.
Clinical semiology of Malassezia dermatitis
Clinical manifestations of Malassezia dermatitis in the French Bulldog present characteristic semiology. Intense erythema, preferentially affecting intertriginous zones (facial folds, interdigital spaces, axial and inguinal folds), is accompanied by severe pruritus and particular rancid odor resulting from lipid degradation by fungal lipases. Affected skin presents shiny greasy appearance with sometimes fine adherent desquamation. Post-inflammatory hyperpigmentation occurs rapidly in chronicity zones, conferring brownish aspect to initially erythematous skin. Interdigital spaces, particularly in atopic subjects, become predilection sites with intense erythema, exudation and interdigital cyst formation secondary to deep follicular inflammation. Compulsive paw licking frequently represents the first warning sign, with brown-reddish coloration of white hairs by salivary porphyrins, indicating chronic licking.
Cytological diagnosis and pathological thresholds
Cytological diagnosis of Malassezia dermatitis relies on demonstration of abnormally elevated yeast numbers by microscopic examination. Several sampling techniques prove usable: direct apposition technique by pressing glass slide on erythematous skin, swabbing followed by slide rolling, or scotch-test particularly adapted to interdigital spaces and folds. After rapid staining, microscopic counting at 1000x magnification under immersion allows semi-quantification. The pathological threshold remains controversial, but consensus proposes as significant the presence of more than 10 yeasts per microscopic field on average over 10 fields, or more than 3 to 5 yeasts per field in zones normally poor in Malassezia like the trunk. In French Bulldogs, cytological counts in lesional zones frequently reveal 25 to 50 organisms per field, testifying to massive proliferation.
Recurrent pyoderma problem
Recurrent pyodermas represent a major therapeutic problem in the French Bulldog, defined by occurrence of three or more bacterial pyoderma episodes over 12 months. This recurrence rarely results from initial episode antibiotic treatment failure, but rather from persistence of uncontrolled underlying predisposing factors. Documented underlying causes in French Bulldogs include atopic dermatitis in 68% of cases, hypothyroidism in 8%, Cushing syndrome in 3%, and rare immune deficits. Uncontrolled atopy creates a vicious circle where chronic inflammation, barrier dysfunction and pruritus with self-trauma perpetuate conditions favorable to bacterial infection. Recognition and treatment of this underlying cause proves imperative to break the recurrent infectious cycle.
Deep bacterial folliculitis and furunculosis
Deep bacterial folliculitis, potential evolution of untreated or recurrent superficial folliculitis, reaches the lower hair follicle segment and can extend to subcutaneous tissue as furunculosis. This severe infection is characterized clinically by firm erythematous nodules, painful on palpation, which can evolve toward abscess formation with fistulous tracts draining hemorrhagic or purulent exudate. Body zones with deep follicles like chin region (mental folliculitis or canine acne), interdigital spaces and pressure points are preferentially affected. Histopathological examination reveals follicular wall rupture with follicular content release (bacteria, keratin, sebum) into surrounding dermis, triggering intense pyogranulomatous granulomatous reaction centered on keratinous debris. Presence of epithelioid macrophages, multinucleated giant cells and progressive fibrosis characterizes this chronic inflammation.
Pododermatitis and interdigital cysts
Pododermatitis, inflammation of plantar pads and interdigital spaces, constitutes a particularly problematic localization in the French Bulldog due to limb anatomical conformation and elevated body weight on reduced support surface. French Bulldog pododermatitis often presents multifactorial etiology combining atopic dermatitis, Malassezia dermatitis and secondary bacterial pyoderma. Interdigital spaces become erythematous, edematous and painful, with hemorrhagic blister formation and fistulous tracts in severe cases. Compulsive licking worsens lesions through repeated mechanical trauma and continuous salivary inoculation. Interdigital cysts, fluctuant or firm nodules of interdigital spaces, result from deep folliculitis and furunculosis with encysted abscess formation. Cytological examination of cyst content typically reveals pyogranulomatous inflammation with degenerate neutrophils, activated macrophages and keratinous material, with or without visible bacteria according to evolutionary stage.
Mucocutaneous pyoderma: erosions and ulcerations
Mucocutaneous pyoderma, rare dermatological condition documented in German Shepherds and sporadically reported in other breeds including French Bulldogs, is characterized by erosive and ulcerative inflammation of mucocutaneous junctions, particularly labial, nasal, perianal and genital. This dermatosis presents complex immunological pathophysiology involving hypersensitivity reaction to staphylococcal bacterial antigens combined with functional T suppressor lymphocyte deficit. Lesions begin with erythema and mucocutaneous junction edema, rapidly evolving toward erosions then superficial painful ulcerations with hemorrhagic crust formation. Histopathological examination reveals lichenoid interface dermatitis with basal layer hydropic degeneration, perivascular lymphoplasmacytic infiltrate and keratinocyte apoptosis. Bacterial culture regularly isolates Staphylococcus pseudintermedius, but improvement under antibiotherapy remains partial and transitory, frequently requiring immunomodulator addition.
Module 6: Demodicosis
Juvenile demodicosis
A parasitic dermatosis that has not disappeared
Canine demodicosis results from excessive pathological proliferation of commensal mites of genus Demodex, mainly Demodex canis and more rarely Demodex injai, normally colonizing hair follicles and sebaceous glands of canine skin at low densities. Demodex canis transmission occurs vertically from dam to newborn puppies by direct contact during first lactation days, mites migrating from maternal mammary glands toward hair follicles of newborns’ face and forelimbs. In most dogs, mite populations remain in homeostatic equilibrium at densities below pathological threshold throughout existence, maintained under control by local innate and adaptive immune defenses. In a minority of individuals, rupture of this equilibrium allows excessive mite proliferation generating inflammatory cutaneous lesions. This homeostatic rupture occurs either by genetically determined primary immune dysfunction in juvenile demodicosis, or by acquired secondary immunosuppression in adult demodicosis.
British VetCompass epidemiological analysis conducted on 455,553 dogs in primary veterinary care during 2013 reveals a global annual prevalence of 0.17% with 95% confidence interval of 0.16 to 0.19% for demodicosis in the general canine population. Age distinction demonstrates dramatically superior prevalence in young animals with 0.48% and confidence interval of 0.45 to 0.52% for dogs under 2 years, compared to 0.05% and confidence interval of 0.04 to 0.06% for dogs over 4 years, ratio illustrating that juvenile demodicosis occurs approximately ten times more frequently than adult form. Among 702 cases for which age at first diagnosis was available, median age was established at 7 months with interquartile interval of 4 to 13 months, 72.4% of cases were under 1 year, 79.6% under 1.5 years, 81.6% under 2 years and only 14.0% over 4 years. This age distribution confirms massive predominance of juvenile presentation of canine demodicosis in primary veterinary practice.
The French Bulldog manifests significant breed susceptibility to juvenile demodicosis with adjusted odds ratio of 5.07 and confidence interval of 3.37 to 7.63 compared to crossbred dogs in multivariate model including breed, weight relative to breed average, age and sex-sterilization status with veterinary clinic as random effect. This predisposition places the French Bulldog sixth among high-risk breeds after English Bulldog with odds ratio of 11.26, Staffordshire Bull Terrier with odds ratio of 7.11, Chinese Shar-Pei with odds ratio of 6.57, Dogue de Bordeaux with odds ratio of 5.92 and Pug with odds ratio of 5.41. Crude annual prevalence of demodicosis in French Bulldogs under 2 years reaches 1.88% with confidence interval of 1.30 to 2.73%, four times prevalence observed in crossbreds of 0.37% and two to three times higher than Boxers. Remarkably, no demodicosis case was identified in French Bulldogs over 4 years in this cohort, suggesting either individuals presenting genetic susceptibility manifest their disease early or French Bulldogs affected by juvenile demodicosis are predominantly cured and do not develop recurrent form in adulthood.
Common membership of English Bulldog, French Bulldog, Staffordshire Bull Terrier, Dogue de Bordeaux and Boxer to the same genetic clade identified by pangenomic phylogenetic analyses strongly suggests shared genetic basis for juvenile demodicosis susceptibility. This clade, grouping bulldog and bull terrier type breeds, shares common ancestral genetic variants resulting from historical population bottlenecks and intensive selection on common morphological characteristics including brachycephaly, stocky body and temperament. Convergent observation of independent American and British epidemiological studies identifying the same breeds of this genetic clade among most predisposed reinforces hypothesis of substantial genetic determinism for juvenile demodicosis. Precise nature of causal variants, their transmission mode and penetrance remain however unelucidated. Current consensus postulates complex polygenic inheritance mode involving multiple loci with moderate effect rather than Mendelian monogenic mutation, coherent with phenotypic variability observed within affected litters.
An immune dysfunction above all
Pathophysiology of juvenile demodicosis involves immune dysfunction specific to Demodex mites, affected dogs manifesting normal immune responses toward other pathogens. Immunological investigations have revealed quantitative and qualitative perturbations of T lymphocyte subpopulations in demodectic dogs. A functional study demonstrated that T lymphocytes from generalized demodectic dogs presented reduced blastic proliferation in response to phytohemagglutinin and concanavalin A stimulation compared to healthy controls, suggesting T lymphocyte reactivity deficit. CD4 positive and CD8 positive lymphocyte subpopulation ratios proved altered with reduced proportion of CD4 positive T helper lymphocytes. Cytokine production by stimulated lymphocytes manifested imbalance toward Th2 polarization with increased secretion of immunosuppressive interleukin-4 and interleukin-10 at expense of pro-inflammatory interferon-gamma and interleukin-2 of Th1 type, inadequate polarization for control of intrafollicular parasitic infestation requiring robust cytotoxic cellular response.
A very pleomorphic dermatosis
Clinical presentation of canine demodicosis is declined according to two complementary classifications. Age classification distinguishes juvenile form typically beginning between 3 and 18 months from adult form appearing after. Lesional extent classification differentiates localized form, characterized by less than five focal lesions of diameter inferior to 2.5 centimeters with favorable prognosis of spontaneous resolution in 90% of cases without acaricide treatment, from generalized form defined by more than five lesions, involvement of more than two anatomical regions, pododemodicosis affecting at least two paws, or extensive lesions covering total body surface superior to that of one limb. Generalized demodicosis invariably requires systemic acaricide treatment and carries reserved prognosis. Primary cutaneous lesions consist of non-inflammatory multifocal to coalescent alopecias initially predominating in peri-ocular regions, labial commissures, internal ear pavilion surface and limbs, anatomical zones where initial mite colonization of newborn puppies is maximal.
In complicated forms, frequent in French Bulldogs due to habitual coexistence with atopic dermatitis and cutaneous folds, massive demodectic infestation breaks follicular cutaneous barrier and favors severe secondary bacterial infections. Bacterial folliculitis then furunculosis by Staphylococcus pseudintermedius generate pustules, erythematous papules, crusts, sero-purulent exudation and intense local pain. Deep forms evolve toward dermal and hypodermal cellulitis with massive edema, fistulization, purulent drainage and satellite lymphadenomegaly. Interdigital demodectic pododermatitis, particularly frequent and refractory, associates violaceous erythema, interdigital space tumefaction, draining fistulas, palmoplantar hyperkeratosis and support lameness due to pain. Chronicity induces hyperpigmentation, lichenification, dermal fibrosis and irreversible cicatricial alopecia in neglected cases.
Rapid and simple diagnosis
Diagnosis of demodicosis relies on microscopic identification of abnormal numbers of Demodex mites in deep cutaneous scrapings. Standardized technique requires vigorous compression of cutaneous fold between thumb and index for 10 to 15 seconds to expel mites from follicle depths, immediately followed by scraping perpendicular to hair direction with scalpel blade previously coated with mineral oil, continued until capillary oozing, guaranteeing sufficient sampling depth reaching dermo-epidermal junction. Scraped material is transferred to slide, covered with coverslip and examined under microscope at 40x and 100x magnifications to count characteristic fusiform adults, nymphs, hexapod larvae and operculated eggs. Minimum five scrapings from distinct lesional sites should be performed to maximize diagnostic sensitivity. Trichogram, consisting of multiple hair plucking with forceps followed by microscopic examination after mounting between slide and coverslip in oil, constitutes less invasive alternative particularly useful for peri-ocular and interdigital localizations difficult to scrape.
The isoxazoline revolution
Therapeutic protocols for canine demodicosis have been revolutionized by advent of oral isoxazolines, pharmacological class of ectoparasiticides modulating GABA and glutamate-dependent chloride channels of invertebrates, inducing paralysis and death of arthropod parasites.
Adult demodicosis, rare but not to be neglected
Adult demodicosis management imperatively requires identification and treatment of underlying immunosuppression causes. Endocrine conditions including hypothyroidism, spontaneous or iatrogenic hypercorticism, and diabetes mellitus should be systematically screened by appropriate hormonal assays. Neoplasias, particularly lymphoma and mastocytoma, and systemic autoimmune diseases constitute other immunosuppression causes favoring adult demodicosis. Immunosuppressive treatments including systemic glucocorticoids, ciclosporin and chemotherapeutic agents must be discontinued if possible.
Module 7: Multimodal diagnostic approach
Clinical methodology and DAMN-IT system
Dermatological diagnosis in the French Bulldog requires methodical approach integrating detailed anamnesis, rigorous clinical examination and targeted complementary examinations. The complexity of dermatological presentation in this breed, frequently combining several superposed pathologies (primary atopy, Malassezia dermatitis, secondary pyoderma, intertrigo), requires practitioner capacity for sequential analysis to identify initial primary condition and its secondary complications. Structured diagnostic approach according to DAMN-IT method (Developmental, Allergic, Metabolic, Neoplastic, Inflammatory, Traumatic, Infectious, Immunologic) allows elaboration of exhaustive differential list before progressively restricting it by elimination or diagnostic confirmation.
Anamnesis: pillar of diagnostic reasoning
Anamnesis constitutes the cornerstone of diagnostic reasoning in dermatology. Meticulous owner interrogation must document several essential temporal and contextual parameters. Age of first clinical sign appearance significantly orients differential diagnosis: early onset before 6 months suggests congenital or hereditary dermatosis, while onset between 6 months and 3 years strongly evokes atopic dermatitis. Symptom seasonality differentiates seasonal atopy linked to pollens from perennial atopy induced by dust mites or food allergens. Pruritic character of condition, evaluated by standardized questionnaire or visual analog scale, represents major diagnostic criterion: pruritus sine materia (pruritus without visible primary lesion) orients toward allergic hypersensitivity, while primary lesions without pruritus suggest metabolic or neoplastic dermatosis. Living environment (urban/rural), housing mode (indoor/outdoor), exposure to other animals and travel history document risk of exposure to ectoparasites or infectious agents.
Systematic clinical examination and otoscopy
Dermatological clinical examination of French Bulldog follows systematic methodology beginning with distance observation of general condition, behavior and pruritus signs (licking, nibbling, rubbing). Close examination evaluates lesion distribution according to standardized anatomical schema, distinguishing primary lesions (macules, papules, pustules, vesicles, nodules) from secondary lesions (erosions, ulcers, crusts, scales, hyperpigmentation, lichenification). Palpation appreciates cutaneous thickening, subcutaneous edema and painful sensitivity of affected zones. Apparent mucous membrane examination (conjunctival, oral, genital) searches for lesion extension to mucocutaneous junctions. Bilateral otoscopic inspection evaluates external auditory canal and tympanic membrane condition, external otitis being present in 75 to 80% of atopic French Bulldogs. Regional lymph node palpation (submandibular, pre-scapular, popliteal) detects reactional lymphadenomegaly translating inflammation or cutaneous infection of drained territory.
Cutaneous cytology: cellular and microbial identification
Cutaneous cytological examination represents first-line complementary examination in veterinary dermatology, practicable in consultation without sophisticated equipment. Several sampling techniques apply according to lesion type. Direct apposition technique on glass slide is used for erythematous or exudative surfaces. Scotch-test, particularly adapted to interdigital spaces and folds, consists of applying transparent adhesive tape piece to examination zone then sticking it to slide. Swabbing followed by slide rolling allows exploration of auricular canals or deep lesions. Fine needle aspiration (cytoponction) of nodules or cysts samples cellular content for analysis. After air drying, slides undergo rapid staining like Diff-Quik (May-Grünwald-Giemsa equivalent) allowing cellular differentiation. Microscopic examination at 400x then 1000x magnification under immersion characterizes inflammatory cellular population (neutrophils, eosinophils, macrophages, lymphocytes), identifies microorganisms (bacterial cocci or bacilli, Malassezia yeasts, rare dermatophytes) and detects atypical cells suggesting neoplasia.
Cutaneous scraping and parasitic diagnosis
Cutaneous scraping, essential parasitological examination, searches for mites responsible for scabies. Two scraping types are performed according to sought depth. Superficial scraping, performed until light erythema appearance, detects Sarcoptes scabiei, mite responsible for sarcoptic scabies characterized by intense recalcitrant pruritus. Deep scraping, continued until capillary oozing, reveals Demodex canis, follicular commensal mite which can proliferate during demodicosis. Although French Bulldog does not figure among breeds at high risk of generalized demodicosis (contrary to Shar-Pei or Bull Terrier), cases of localized peri-ocular or lip demodicosis occasionally occur. Scraping material, transferred to slide with mineral oil or lactophenol drop, is examined under microscope at 100x then 400x magnification. Identification of mite adults, larvae, nymphs or eggs confirms parasitic diagnosis.
Trichoscopy: hair shaft and cycle analysis
Trichoscopy or microscopic hair examination completes diagnostic arsenal for alopecias. Hair sampling by gentle plucking with hemostatic forceps in alopecic zones allows hair shaft and bulb analysis. Microscopic examination at low then high magnification evaluates hair cycle phase (anagen with pigmented bulb and epithelial sheath, telogen with club bulb without sheath), searches for shaft structural anomalies (trichorrhexis nodosa, trichomalacia) and identifies fungal spores in dermatophytosis. In French Bulldog, alopecia most often results from pruritus with mechanical hair plucking (traumatic alopecia), and trichoscopy then reveals hair shafts fractured at irregular extremities without bulb anomaly, contrasting with endocrine alopecias where hairs epilate easily with telogen bulbs.
Allergological tests: IDT and serum IgE assays
Allergological tests are indicated in French Bulldogs after atopic dermatitis diagnosis confirmation according to Favrot criteria, to identify involved allergens and consider specific immunotherapy. Two complementary approaches coexist: intradermal tests (IDT) and specific serum IgE assays. Intradermal test represents gold standard, injecting intradermally at clipped thorax level a battery of standardized allergens (mites, pollens, molds, epithelials) diluted in saline solution. Reading after 15 to 20 minutes quantifies papular and erythematous reaction compared to positive (histamine) and negative (physiological solution) controls. Positive result is defined by papule diameter superior to 50% of histamine reaction. Serological specific IgE allergen assays by ELISA or immunoblot technique constitute less invasive alternative, although their sensitivity and specificity remain inferior to IDT. Imperfect concordance exists between these two methods, with agreement in only 60 to 70% of cases, justifying clinical interpretation of results rather than mechanical application.
Elimination diet and food provocation test
Food elimination diet represents the only reliable diagnostic test to confirm or exclude food hypersensitivity contributive to clinical presentation. This diet consists of exclusively feeding dog for 8 to 12 weeks with protein and carbohydrate source to which animal has never been exposed (novel protein diet) or with hydrolyzed food whose proteins fragmented into low molecular weight peptides (<10 kDa) lose their allergenicity. Strict observance constitutes major limiting factor of this test, any dietary infraction (treats, table scraps, flavored medications) invalidating result. Clinical improvement superior to 50% of pruritus and cutaneous lesions under diet strongly evokes food component. Diagnostic confirmation requires provocation test by reintroduction of previous diet, which should trigger relapse within 7 to 14 days. If relapse occurs, progressive reintroduction of individual ingredients allows identification of specific responsible allergen(s).
Histopathological examination and cutaneous biopsies
Cutaneous histopathological examination, although non-specific for atopic dermatitis, is indicated during atypical clinical presentation, inadequate therapeutic response or suspicion of neoplastic or autoimmune process. Cutaneous biopsies with 6 to 8 millimeter punch are performed under local or general anesthesia, sampling representative primary lesions avoiding zones of extreme chronicity or superinfection. Sampling lesions at different evolutionary stages and including peripheral healthy skin sample optimize diagnostic information. Samples fixed in 10% buffered formalin undergo standard histotechnical treatment with paraffin inclusion, cutting and hematoxylin-eosin staining. Microscopic analysis by veterinary dermatopathologist characterizes lesional architecture, inflammatory infiltrate type and distribution, and searches for etiological indices. Atopic dermatitis typically presents superficial perivascular dermatitis with lympho-histiocytic and eosinophilic infiltrate, epidermal hyperplasia (acanthosis), orthokeratotic hyperkeratosis and variable spongiosis according to lesional acuity.
Module 8: Integrated therapeutic strategies and perspectives
Objectives of multimodal management
Therapeutic management of atopic dermatitis and associated dermatological complications in French Bulldogs requires multimodal and individualized approach combining secondary infection control, anti-inflammatory and anti-pruritic treatment, cutaneous barrier restoration, and environmental factor management. Realistic therapeutic objective aims not for definitive cure of this chronic dermatosis, but satisfactory symptom control allowing acceptable quality of life for animal and owner, with minimization of long-term side effects and economic cost. Therapeutic strategy evolves according to initial severity, treatment response and comorbidity presence, requiring regular re-evaluations and posological or molecular adjustments.
Secondary infection treatment and antibiotherapy
Secondary infection treatment imperatively constitutes first therapeutic step before evaluating anti-inflammatory response. Superficial pyodermas by methicillin-sensitive Staphylococcus pseudintermedius generally respond to systemic antibiotherapy with first-generation cephalosporins (cephalexin 15 to 25 mg/kg orally twice daily) or amoxicillin-clavulanic acid (12.5 to 25 mg/kg orally twice daily) for minimum 21 to 28 days, one week beyond complete clinical resolution. Deep pyodermas or MRSP strains require prolonged antibiotherapy of 6 to 8 weeks with broad-spectrum molecules: fluoroquinolones (enrofloxacin 5 to 10 mg/kg once daily, marbofloxacin 2.75 to 5.5 mg/kg once daily) after bacterial culture with antibiogram. Growing resistance imposes rational antibiotic use according to prudent antibiotherapy recommendations, privileging topical treatments when possible.
Topical antiseptic use and chlorhexidine
Topical antiseptics represent valuable alternative or complement to systemic antibiotherapy, particularly for localized or recurrent infections. Chlorhexidine, cationic biguanide with broad bactericidal and fungicidal spectrum, is used as shampoo at 2 to 4% concentration with 10-minute contact time before rinsing, two to three weekly applications. Its mechanism of action relies on bacterial membrane disorganization by binding to anionic phospholipids. Controlled clinical studies demonstrate 3% chlorhexidine efficacy comparable to systemic antibiotics for localized superficial pyodermas, with advantage of avoiding systemic resistance selection. Formulations combining chlorhexidine and miconazole (azole antifungal) simultaneously treat bacterial and Malassezia infections, frequently intertwined in French Bulldogs. Chlorhexidine-impregnated wipes facilitate daily facial fold antisepsis, preventing intertrigo.
Janus kinase inhibitors
Janus kinase inhibitors (JAK inhibitors) represent major therapeutic advance in veterinary dermatology since oclacitinib market authorization in 2013 for canine atopic pruritus symptomatic treatment. Oclacitinib, synthetic small molecule administered orally, selectively inhibits JAK1 and JAK3 tyrosine kinases (and to lesser extent JAK2), thus blocking signal transduction of multiple pro-pruritic and pro-inflammatory cytokines including IL-31, IL-4, IL-13 and IL-2. Recently new Janus kinase inhibitors have been marketed, namely ilunocitinib (Zenrelia) and atinvicitinib (Numelvi), each presenting various advantages and disadvantages.
Lokivetmab: anti-IL-31 monoclonal antibody
Lokivetmab, caninized canine monoclonal antibody specifically directed against interleukin-31, constitutes biotherapeutic alternative to oclacitinib. This therapeutic antibody, produced by recombinant technology, neutralizes circulating IL-31 by high-affinity binding, preventing its interaction with IL-31RA/OSMR receptor complex. Administration is by subcutaneous route at 1 to 2 mg/kg dosage, with prolonged action duration of 4 to 8 weeks allowing monthly injections. Pivotal phase III studies reveal pruritus reduction of more than 50% in 79% of treated dogs after first injection, with median action delay of 1.2 days. Lokivetmab major advantage resides in its extreme immunological specificity, targeting only IL-31 without interfering with other signaling pathways, thus minimizing systemic side effects. Its canine immunoglobulin protein nature limits immunogenicity risk with anti-drug antibody formation, contrary to non-caninized recombinant proteins.
Ciclosporin and calcineurin inhibition
Ciclosporin, calcineurin inhibitor immunomodulator, historically represents first validated systemic treatment for canine atopic dermatitis with market authorization since 2003. This cyclic lipophilic molecule derived from Tolypocladium inflatum fungus inhibits T lymphocyte activation by calcineurin phosphatase blockade, preventing NFAT (Nuclear Factor of Activated T-cells) transcription factor dephosphorylation and nuclear translocation. Pro-inflammatory cytokine IL-2, IL-4, IL-5, IL-13 and TNF-α transcriptional cascade is thus blocked. Initial dosage of 5 mg/kg orally once daily, administered fasting to optimize absorption, continues for 4 to 6 weeks until obtaining satisfactory clinical response, then gradually reduces to minimum effective dose, often 2.5 to 5 mg/kg every 2 to 3 days in maintenance. Gastrointestinal side effects (vomiting, diarrhea, anorexia) occur in 20 to 30% of dogs, mainly during first weeks, and generally attenuate spontaneously or by administration with light meal despite reduced absorption.
Glucocorticoid place in acute flares
Systemic glucocorticoids, potent rapid-acting anti-inflammatories, maintain place in severe acute flare emergency treatment despite long-term side effects. Prednisolone or prednisone is administered at anti-inflammatory dose of 0.5 to 1 mg/kg orally once to twice daily until pruritus and inflammation control (generally 7 to 14 days), followed by progressive decrease over 2 to 3 weeks then complete weaning or transition to alternative treatment. Chronic daily corticoid use inevitably leads to iatrogenic Cushing syndrome with polyuria-polydipsia, polyphagia, amyotrophy, hepatomegaly and immunosuppression favoring opportunistic infections. Alternate-day regimen with every-other-day administration partially limits hypothalamic-pituitary-adrenal axis suppression. Long-acting injectable corticoids (methylprednisolone acetate) are formally discouraged in dermatology due to impossibility of posological adjustment and increased risk of severe side effects.
Role of emollients and restructuring topicals
Emollient and barrier restructuring topicals play essential adjuvant therapeutic role often underestimated. Formulations enriched with physiological ceramides, cholesterol and essential fatty acids in equimolar ratio mimicking stratum corneum lipid composition partially restore deficient barrier function. A prospective randomized controlled clinical study evaluating restructuring spray containing ceramide complex, fatty acids and phytosphingosine in 60 atopic dogs demonstrates significant CADESI score reduction of 42% after 8 weeks of twice-daily application, associated with 38% decrease in trans-epidermal insensible water loss. Therapeutic shampoos based on colloidal oatmeal, omega-6 fatty acids and glycerin exert immediate antipruritic and moisturizing effect, with optimal benefit during twice-weekly use with 10-minute contact time. Soap-free formulations (syndets or synthetic detergents) at physiological acid pH preserve cutaneous lipid film contrary to conventional alkaline shampoos.
Allergen-specific immunotherapy (desensitization)
Allergen-specific immunotherapy (desensitization or hyposensitization) represents the only potentially disease-modifying treatment for atopic disease, aiming to induce immunological tolerance to identified allergens rather than simply suppressing symptoms. Classic subcutaneous protocol begins with induction phase with weekly injections of allergenic extracts at increasing concentrations over 16 to 20 weeks, followed by maintenance phase with monthly injections continued for minimum 12 months, ideally 2 to 3 years. Involved immunological mechanisms include Th2 to Th1 response deviation, induction of regulatory T lymphocytes producing immunosuppressive IL-10 and TGF-β, and isotype switch from IgE production to blocking IgG4. Success rates reported in veterinary literature vary considerably according to studies, with excellent to good improvement in 60 to 75% of dogs, moderate improvement in 15 to 20%, and failure in 10 to 15%. Optimal response often requires 9 to 12 months of treatment before definitive evaluation. Regional intradermal protocols and sublingual formulations emerge as potentially safer and better-tolerated alternatives.
Impact of polyunsaturated fatty acids (Omega-3 and 6)
Long-chain omega-3 and omega-6 polyunsaturated fatty acids exert anti-inflammatory and barrier restructuring effects through several mechanisms. Eicosapentaenoic acid (EPA, C20:5 ω-3) and docosahexaenoic acid (DHA, C22:6 ω-3) contained in fish oils incorporate into inflammatory cell membrane phospholipids, partially substituting for arachidonic acid (C20:4 ω-6). During cellular activation, phospholipase A2 releases these fatty acids which are metabolized by cyclooxygenases and lipoxygenases into series 3 and 5 mediators (prostaglandins PGE3, leukotrienes LTB5) less pro-inflammatory than their series 2 and 4 homologs derived from arachidonic acid. High-dose supplementation (75 to 150 mg/kg EPA+DHA per day) for minimum 8 to 12 weeks exerts moderate effect on atopic pruritus, with average 20 to 30% reduction in controlled studies, justifying their use as therapeutic adjuvant but not in monotherapy.
Future therapeutic perspectives and gene therapy
Future therapeutic perspectives in canine atopic dermatology orient toward increasingly specific molecular targets. Dupilumab, human monoclonal antibody targeting IL-4 receptor alpha subunit and thus blocking IL-4 and IL-13 signaling, currently revolutionizes severe human atopic dermatitis treatment with response rates superior to 70%. Development of caninized canine equivalent represents promising avenue. Topical JAK inhibitors (ruxolitinib, tofacitinib in dermal formulations) would allow targeted cutaneous pro-inflammatory signaling blockade without systemic immunosuppression. Probiotics and prebiotics aiming to modulate cutaneous and intestinal microbiome are subject of intensive research, certain Lactobacillus strains demonstrating in vitro immunomodulatory properties with regulatory T lymphocyte induction. Gene therapy by functional FLG gene transfer into keratinocytes remains experimental but could theoretically correct primary filaggrin deficit. Therapeutic vaccines based on immunomodulatory peptides derived from allergen but devoid of IgE-reactive epitopes could induce tolerance without anaphylactic risk.
Conclusion
The French Bulldog presents exceptional dermatological vulnerability resulting from unique convergence of anatomical, genetic and immunological factors. Extreme brachycephalic conformation engenders pathological cutaneous folds predisposing to intertrigo and microbial superinfections. Documented genetic anomalies affecting the PROM1 gene and epidermal barrier protein expression compromise stratum corneum cohesion and increase transcutaneous allergen permeability. Primary barrier dysfunction is characterized by filaggrin deficiency, ceramide depletion and tight junction alteration, creating the pathophysiological substrate for atopic dermatitis whose prevalence reaches 15 to 20% in this breed population.
Complex immunological pathogenesis involves excessive Th2 polarization with IL-4, IL-13, IL-5 and IL-31 hyperproduction, leading to massive allergen-specific IgE synthesis and tissue eosinophilic recruitment. Cutaneous dysbiosis with Staphylococcus pseudintermedius and Malassezia pachydermatis expansion amplifies inflammation through superantigenic exotoxin and proteolytic enzyme production. Secondary infectious complications, notably recurrent pyodermas and Malassezia dermatitis, constitute the rule rather than exception, requiring rigorous antimicrobial management prior to any anti-inflammatory response evaluation.
Diagnostic approach relies on structured clinical methodology integrating detailed anamnesis, systematic dermatological examination and targeted complementary examinations, particularly cutaneous cytology practicable as first-line investigation. Secondary infection identification and appropriate treatment imperatively constitute first therapeutic step. Long-term control strategy combines pruritus symptomatic treatment by JAK inhibitors or anti-IL-31 antibodies, epidermal barrier restoration by restructuring topicals, and triggering environmental factor management.
Future research perspectives must deepen breed genetic determinism characterization through genome-wide association studies, identify specific variants of epidermal barrier genes predisposing to atopy in French Bulldogs, and develop targeted therapies correcting these primary molecular deficits. Cutaneous microbiome exploration by metagenomic sequencing and identification of dysbiotic signatures predictive of clinical evolution could allow early interventions through probiotics or microbial transplantation. Development of canine biotherapeutics targeting key Th2 cytokines, notably anti-IL-4Rα or anti-IL-13 monoclonal antibodies, opens revolutionary therapeutic perspectives. Finally, early preventive strategies aimed at restoring epidermal barrier from first months of life in at-risk puppies merit thorough investigation to determine if neonatal intervention could prevent or delay clinically manifest atopic dermatitis installation.
References
Outerbridge CA, Jordan TJM. Current Knowledge on Canine Atopic Dermatitis: Pathogenesis and Treatment. Advances in Small Animal Care. 2021;2(1):101-117. PMC9204668.
Bajwa J. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. Canadian Veterinary Journal. 2015;56(8):758-765. PMC4531508.
Zur G, Ihrke PJ, White SD, Kass PH. Canine atopic dermatitis: a retrospective study of 266 cases examined at the University of California, Davis, 1992-1998. Part I. Clinical features and allergy testing results. Veterinary Dermatology. 2002;13(2):89-102.
Marsella R, Olivry T, Nicklin C, Lopez J. Pilot investigation of a model for canine atopic dermatitis: environmental house dust mite challenge of high-IgE-producing beagles, mite hypersensitive dogs with atopic dermatitis and normal dogs. Veterinary Dermatology. 2006;17(1):24-35.
Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Veterinary Research. 2015;11:210. PMC4531508.
Chermprapai S, Broere F, Gooris G, Schlotter YM, Rutten VPMG, Bouwstra JA. Altered lipid properties of the stratum corneum in Canine Atopic Dermatitis. Biochim Biophys Acta Biomembr. 2018;1860(2):526-533.
Pucheu-Haston CM, Bizikova P, Eisenschenk MN, Santoro D, Nuttall T, Marsella R. Review: The role of antibodies, autoantigens and food allergens in canine atopic dermatitis. Veterinary Dermatology. 2015;26(2):115-e30.
Santoro D, Marsella R, Pucheu-Haston CM, et al. Review: Pathogenesis of canine atopic dermatitis: skin barrier and host-micro-organism interaction. Veterinary Dermatology. 2015;26(2):84-e25.
Banovic F, Linder KE, Olivry T. Clinical, microscopic and microbial characterization of exfoliative superficial pyoderma-associated epidermal collarettes in dogs. Veterinary Dermatology. 2017;28(1):107-e23.
Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Veterinary Research. 2015;11:196. PMC4531508.
Cosgrove SB, Wren JA, Cleaver DM, et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Veterinary Dermatology. 2013;24(6):587-597.
Gonzales AJ, Humphrey WR, Messamore JE, et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Veterinary Dermatology. 2013;24(1):48-e12.
Michels GM, Ramsey DS, Walsh KF, et al. A blinded, controlled study to investigate the efficacy and safety of a canine anti-IL-31 monoclonal antibody in client-owned dogs with atopic dermatitis. Veterinary Dermatology. 2016;27(6):505-e136.
Olivry T, Bizikova P. A systematic review of randomized controlled trials for prevention or treatment of atopic dermatitis in dogs: 2008-2011 update. Veterinary Dermatology. 2013;24(1):97-e26.
Marsella R, Girolomoni G. Canine models of atopic dermatitis: a useful tool with untapped potential. Journal of Investigative Dermatology. 2009;129(10):2351-2357.
Banovic F, Olivry T, Bazzle L, Tobias JR, Atlee B, Linder KE. Deep pyoderma caused by Pseudomonas aeruginosa in dogs: 20 cases. Veterinary Dermatology. 2016;27(6):494-e131.
Little PR, King VL, Davis KR, et al. A blinded, controlled multicenter study to assess the effectiveness and rate of flea kill of orally administered afoxolaner compared with topically applied fipronil/(S)-methoprene in dogs with flea allergy dermatitis. Veterinary Parasitology. 2015;207(3-4):306-312.
Loeffler A, Cobb MA, Bond R. Comparison of a chlorhexidine and a benzoyl peroxide shampoo as sole treatment in canine superficial pyoderma. Veterinary Record. 2011;169(10):249.
Mueller RS, Bergvall K, Bensignor E, Bond R. A review of topical therapy for skin infections with bacteria and yeast. Veterinary Dermatology. 2012;23(4):330-e62.
Noli C, Borio S, Varina A, Schievano C. Development and validation of a questionnaire to evaluate the Quality of Life of cats with skin disease and their owners, and its use in 185 cats with skin disease. Veterinary Dermatology. 2016;27(4):247-e58.
Nuttall TJ, Marsella R, Rosenbaum MR, Gonzales AJ, Fadok VA. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. Journal of the American Veterinary Medical Association. 2019;254(11):1291-1300.
Olivry T, Mueller RS, International Task Force on Canine Atopic Dermatitis. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Veterinary Dermatology. 2003;14(3):121-146.
Piekutowska A, Pin D, Reme CA, Gatto H, Haftek M. Effects of a topically applied preparation of epidermal lipids on the stratum corneum barrier of atopic dogs. Journal of Comparative Pathology. 2008;138(4):197-203.
Reedy LM, Miller WH, Willemse T. Allergic Skin Diseases of Dogs and Cats. 2nd ed. London: WB Saunders; 1997.
Saridomichelakis MN, Koutinas AF, Farmaki R, Leontides LS, Kasabalis D. Relative sensitivity of hair pluckings and exudate microscopy for the diagnosis of canine demodicosis. Veterinary Dermatology. 2007;18(2):138-141.
Shimada K, Yoon JS, Yoshihara T, Iwasaki T, Nishifuji K. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Veterinary Dermatology. 2009;20(5-6):541-546.
Shumaker AK, Angus JC, Coyner KS, Loeffler DG, Van Den Broek AHM, Sánchez A. Microbiological and histopathological features of canine pyotraumatic dermatitis (“hot spots”). Veterinary Dermatology. 2008;19(3):131-139.
Steffan J, Alexander D, Brovedani F, Fisch RD. Comparison of cyclosporine A with methylprednisolone for treatment of canine atopic dermatitis: a parallel, blinded, randomized controlled trial. Veterinary Dermatology. 2003;14(1):11-22.
Steffan J, Favrot C, Mueller R. A systematic review and meta-analysis of the efficacy and safety of cyclosporin for the treatment of atopic dermatitis in dogs. Veterinary Dermatology. 2006;17(1):3-16.
Thyssen JP, Godoy-Gijon E, Elias PM. Ichthyosis vulgaris: the filaggrin mutation disease. British Journal of Dermatology. 2013;168(6):1155-1166.
Wilhem S, Kovalik M, Favrot C. Breed-associated phenotypes in canine atopic dermatitis. Veterinary Dermatology. 2011;22(2):143-149.
Bizikova P, Papiernik D, Mendoza ME, Haile B, Olivry T. Studies Using Antibodies against Filaggrin and Filaggrin 2 in Canine Normal and Atopic Skin Biopsies. Veterinary Sciences. 2024;11(2):53. PMC10854974.
Zur G, White SD, Ihrke PJ, Kass PH, Toebe N. Canine atopic dermatitis: a retrospective study of 169 cases examined at the University of California, Davis, 1992-1998. Part II. Response to hyposensitization. Veterinary Dermatology. 2002;13(2):103-111.
Marsella R, Samuelson D, Doerr K. Transmission electron microscopy studies in an experimental model of canine atopic dermatitis. Veterinary Dermatology. 2010;21(1):81-88.
Marsella R, Olivry T, Carlotti DN. Current evidence of skin barrier dysfunction in human and canine atopic dermatitis. Veterinary Dermatology. 2011;22(3):239-248.
Nuttall T, Knight PA, McAleese SM, Lamb JR, Hill PB. Expression of Th1, Th2 and immunosuppressive cytokine gene transcripts in canine atopic dermatitis. Clinical & Experimental Allergy. 2002;32(5):789-795.
Bruet V, Bourdeau PJ, Roussel A, Imparato L, Desfontis JC. Characterization of pruritus in canine atopic dermatitis, flea bite hypersensitivity and flea infestation and its role in diagnosis. Veterinary Dermatology. 2012;23(6):487-e93.
Olivry T, Dunston SM, Murphy KM, Moore PF. Characterization of the inflammatory infiltrate during IgE-mediated late phase reactions in the skin of normal and atopic dogs. Veterinary Dermatology. 2001;12(1):49-58.
Sousa CA, Marsella R. The ACVD task force on canine atopic dermatitis (II): genetic factors. Veterinary Immunology and Immunopathology. 2001;81(3-4):153-157.
DeBoer DJ, Marsella R. The ACVD task force on canine atopic dermatitis (XII): the relationship of cutaneous infections to the pathogenesis and clinical course of canine atopic dermatitis. Veterinary Immunology and Immunopathology. 2001;81(3-4):239-249.
Older CE, Diesel A, Patterson AP, Meason-Smith C, Johnson TJ, Rodrigues Hoffmann A. The feline skin microbiota: The bacteria inhabiting the skin of healthy and allergic cats. PLoS One. 2017;12(6):e0178555.
Bradley CW, Morris DO, Rankin SC, et al. Longitudinal evaluation of the skin microbiome and association with microenvironment and treatment in canine atopic dermatitis. Journal of Investigative Dermatology. 2016;136(6):1182-1190.
Rodrigues Hoffmann A, Patterson AP, Diesel A, et al. The skin microbiome in healthy and allergic dogs. PLoS One. 2014;9(1):e83197.
Yeon SC, Erb HN, Houpt KA. A retrospective study of canine house soiling: diagnosis and treatment. Applied Animal Behaviour Science. 1999;66(1-2):81-95.
Nuttal T, Mueller R. Advances in atopic dermatitis. Companion Animal. 2018;23(2):70-77.
Torres SMF, Gomez N, Dent P. Mast cell quantification in skin biopsies from dogs with allergic skin disease: a retrospective study. Veterinary Dermatology. 2012;23(Suppl 1):3-8.
Keppel KE, Campbell KL, Zuckermann FA, Greeley EA, Schaeffer DJ, Husmann RJ. Quantitation of canine regulatory T cell populations, serum interleukin-10 and allergen-specific IgE concentrations in healthy control dogs and canine atopic dermatitis patients receiving allergen-specific immunotherapy. Veterinary Immunology and Immunopathology. 2008;123(3-4):337-344.
Masuda K, Sakaguchi M, Fujiwara S, et al. Positive reactions to common allergens in 42 atopic dogs in Japan. Veterinary Immunology and Immunopathology. 2000;73(2):193-204.
Hill PB, Hillier A, Olivry T. The ACVD task force on canine atopic dermatitis (VI): IgE-induced immediate and late-phase reactions, two inflammatory sequences at sites of intradermal allergen injections. Veterinary Immunology and Immunopathology. 2001;81(3-4):199-204.
Bizikova P, Santoro D, Marsella R, Nuttall T, Eisenschenk MN, Pucheu-Haston CM. Review: Clinical and histological manifestations of canine atopic dermatitis. Veterinary Dermatology. 2015;26(2):79-e24.