Plasma cell pododermatitis is a rare and poorly understood feline dermatosis, which must not be overlooked during consultation, particularly due to the pain and therefore lameness it can cause. An overview of the latest knowledge, in terms of aetiopathogenesis, as well as diagnosis and the most recent published treatments and protocols.
Introduction and nosological framework
1.1. Definition and nomenclature
Feline plasma cell pododermatitis (FPP) constitutes a rare inflammatory dermatosis, characterised by massive infiltration of mature plasma cells in the dermis of the cat’s footpads. This entity, colloquially designated by the anglophone term pillow foot due to the swollen and soft appearance of affected pads, occupies a unique place in the veterinary dermatological landscape (Miller 2013). The disease is distinguished by its exclusive tropism for the paw tissue, with no exact equivalent in dogs or humans, although pathophysiological parallels can be drawn with certain human cutaneous plasma cell proliferations. Nosologically, FPP belongs to the group of feline plasma cell dermatoses, a spectrum that includes chronic plasma cell stomatitis and, more controversially, certain forms of extra-paw plasma cell dermatitis (Gross 2005). The infiltrate is composed of more than 90% well-differentiated plasma cells expressing the CD138 marker (syndecan-1), which formally distinguishes it from plasma cell neoplasias such as extramedullary plasmacytoma, where the Ki-67 proliferation index usually exceeds 20% (Mauldin 2016).
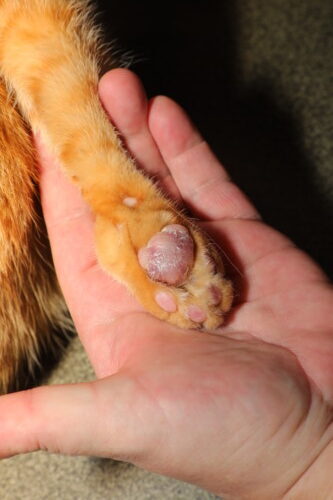
Classic appearance of feline plasma cell pododermatitis
1.2. Historical milestones and evolution of knowledge
The first clinicopathological descriptions of FPP date back to the late 1970s, when Gruffydd-Jones, Orr and Lucke published in 1980 a series of five cats presenting with swelling and ulceration of the pads associated with dense dermal plasma cell infiltration (Gruffydd-Jones 1980). This foundational publication established the basic morphological criteria that still guide recognition of the condition. During the following decade, Taylor and Schmeitzel reported in 1990 two cases complicated by chronic haemorrhages at the level of the pads, highlighting the potential evolution of the disease towards deep ulceration and vascular fragility (Taylor 1990). The advent of serological tests for the feline immunodeficiency virus (FIV) in the 1990s enabled the highlighting of a statistical association between retroviral infections and FPP, a link explored in several retrospective series showing an FIV seroprevalence ranging from 20 to 62% in affected cats, compared with 2 to 4% in the general feline population (Guaguère 2004; Dias Pereira 2003). The retrospective study by Guaguère and collaborators, covering 26 cases, constitutes to date one of the largest published cohorts and has contributed to refining the understanding of the epidemiological profile and therapeutic response (Guaguère 2004). The introduction of doxycycline as first-line treatment, at the beginning of the 2000s, represented a therapeutic turning point, offering an alternative to long-term corticotherapy with a complete response rate approaching 50 to 65% according to the series (Bettenay 2003).
Epidemiology
2.1. Prevalence and incidence data
FPP remains an infrequent condition whose exact prevalence is difficult to establish, partly due to probable underdiagnosis linked to the spontaneous resolution of certain benign forms. Available data, from referral centres in veterinary dermatology, place its frequency between 0.5 and 1.5% of all feline dermatological consultations (Miller 2013). In the series by Dias Pereira and Faustino, analysing 8 cases diagnosed over a seven-year period in a Portuguese university centre, the annual incidence is estimated at approximately 1.1 cases per 1,000 feline consultations (Dias Pereira 2003). This relative rarity does not exclude an underestimated prevalence in routine practice, insofar as early non-ulcerated stages frequently go unnoticed. No significant geographical variation has been reported, with cases documented on all continents, from Europe to North America, through Asia and Australia (Hnilica 2017). The sporadic nature of the disease, without temporal or spatial clustering phenomena, argues against a direct transmissible infectious aetiology and points rather towards an individual dysimmune pathogenesis.
2.2. Demographic risk factors: breed, age and sex
Unlike many feline dermatoses for which a genetic determinism has been identified — for example the PNPLA1 gene in Golden Retriever ichthyosis or the KRT10 gene in epidermolytic ichthyosis — no clear breed predisposition has been demonstrated for FPP (Miller 2013). The majority of reported cases concern domestic short-haired cats, which reflects the predominance of this population in veterinary consultations rather than specific genetic susceptibility. Guaguère’s cohort included 23 European cats out of 26 (88.5%), the remaining three being respectively a Persian, a Siamese and a Chartreux (Guaguère 2004). Analysis of Dias Pereira’s series confirms this absence of breed predisposition, with a distribution proportional to the reference population (Dias Pereira 2003).
The age at diagnosis varies considerably, ranging from 6 months to 14 years, with a median between 4 and 7 years according to studies (Gross 2005; Guaguère 2004). This wide age range suggests that the disease corresponds neither to a juvenile developmental condition, nor to a strictly geriatric pathology, but that it can occur at any time in adult life depending on the convergence of individual immunological factors. Regarding sex, the data remain contradictory. Some series report a slight male predominance with a sex ratio of 1.4:1 in favour of males (Guaguère 2004), whilst others find no significant difference. Reproductive status (entire or neutered) does not appear to exert a determining influence, although most cats included in published studies are neutered, in accordance with the domestic feline population management practices in force in industrialised countries. The absence of an identified genetic polymorphism associated with FPP contrasts with other feline immune dermatoses, such as the eosinophilic granuloma complex where polygenic genetic factors have been suspected, and reinforces the hypothesis of an acquired pathogenesis involving environmental or infectious cofactors.
Aetiopathogenesis: a multifactorial immune dysregulation
3.1. Arguments in favour of a dysimmune origin
The aetiology of FPP remains to date incompletely elucidated, but all clinical, biological and histopathological data converge towards an immune-mediated pathogenesis. Several arguments support this hypothesis. The first rests on the very nature of the infiltrate, composed almost exclusively of mature polyclonal plasma cells, indicators of chronic and sustained antigenic activation of the B lymphocyte compartment. Immunohistochemical analysis reveals that these plasma cells express both kappa and lambda light chains of immunoglobulins in a physiological ratio of approximately 2:1, excluding a neoplastic monoclonal process (Gross 2005; Mauldin 2016). The second argument derives from the polyclonal hypergammaglobulinaemia documented in 50 to 63% of affected cats, testifying to diffuse stimulation of the humoral immune system, without identified antigenic specificity (Guaguère 2004). Serum protein electrophoresis demonstrates an elevation of gamma fractions without monoclonal peak, formally distinguishing this condition from multiple myeloma or secreting plasmacytoma. Finally, the favourable response to immunomodulatory or immunosuppressive agents — doxycycline, glucocorticoids, ciclosporin — constitutes an indirect therapeutic argument in favour of a dysimmune mechanism (Bettenay 2003; Miller 2013).
The detection of antinuclear antibodies (ANA) in a non-negligible proportion of affected cats, estimated between 25 and 50% according to series, reinforces the autoimmune hypothesis, although the clinical significance of these ANA remains debated in feline medicine (Dias Pereira 2003). Their presence could reflect non-specific lymphocyte activation rather than true self-directed reactivity. Direct immunofluorescence analysis of pad biopsies has demonstrated deposits of IgG, IgM and C3 complement fraction at the dermo-epidermal junction and around dermal vessels in a majority of cases, a pattern suggestive of an immune complex disease (Gross 2005). These deposits recall those observed in feline systemic lupus erythematosus, suggesting a partially shared pathophysiological mechanism involving the classical complement pathway triggered by fixation of the C1q fragment to antigen-antibody complexes.
3.2. Involvement of feline immunodeficiency virus and retroviruses
The association between FPP and FIV infection constitutes one of the most discussed aspects of aetiopathogenesis. Several retrospective studies report a significantly higher FIV seroprevalence in cats with FPP than in the general population. In Guaguère’s series, 50% of the 26 tested cats were FIV-seropositive, a rate markedly higher than the expected prevalence of 2 to 4% in the European domestic feline population (Guaguère 2004). Dias Pereira and Faustino found a seroprevalence of 62.5% in their cohort of 8 cases (Dias Pereira 2003). FIV, a lentivirus of the Retroviridae family, causes profound dysregulation of adaptive immunity through progressive depletion of CD4+ T lymphocytes and by chronic polyclonal activation of B lymphocytes. This phenomenon, mediated by direct stimulation of the Toll-like receptor 7 (TLR7) by single-stranded viral RNA and by aberrant secretion of IL-6, promotes plasma cell differentiation and production of non-specific immunoglobulins (Hartmann 2012). Activation of the JAK1/STAT3 pathway downstream of the IL-6 receptor constitutes a key mechanism of this excessive plasmacytogenesis, with the phosphorylated STAT3 transcription factor translocating to the nucleus to induce expression of BLIMP-1 (PRDM1), the master regulator of terminal B lymphocyte differentiation into plasma cells (Nutt 2015).
The association with feline leukaemia virus (FeLV) is less well documented, with seroprevalence rates oscillating between 0 and 20% according to cohorts, figures not always differing significantly from the reference population (Guaguère 2004; Miller 2013). FPP can however occur in cats seronegative for both FIV and FeLV, indicating that retroviral infection is neither necessary nor sufficient to trigger the disease. It probably acts as an amplifying cofactor of pre-existing immune dysregulation, lowering the tolerance threshold and promoting the rupture of B compartment homeostasis.
3.3. Suspected environmental and infectious cofactors
Beyond retroviruses, other infectious agents have been suspected as potential triggers without formal proof being provided. Certain authors have evoked the role of chronic antigenic stimulations linked to bacterial or fungal agents in contact with the pads, but no systematic microbiological culture has demonstrated a specific pathogen associated with the disease (Miller 2013). The hypothesis of contact hypersensitivity or foreign body reaction has been advanced, without experimental support. Cases of FPP occurring after vaccination or dietary change have been reported anecdotally, but no causal link has been established (Scarampella 2004). The role of epigenetic factors modulating the expression of genes involved in B compartment regulation — such as post-translational histone modifications or DNA methylation at PRDM1 and IRF4 promoter levels — remains an unexplored field of research in feline medicine.
Molecular immunopathology
4.1. Plasma cell ontogeny and involved signalling pathways
Understanding FPP requires detailed knowledge of plasma cell biology. Terminal differentiation of the B lymphocyte into a mature immunoglobulin-secreting plasma cell is orchestrated by a hierarchical network of transcription factors. The BLIMP-1 factor, encoded by the PRDM1 gene, acts as a transcriptional repressor of the B cell programme, inhibiting expression of PAX5 and BCL6, two factors essential for maintaining germinal centre B lymphocyte identity (Nutt 2015). Simultaneously, the IRF4 factor (MUM1) binds in synergy with BLIMP-1 to activate the secretory programme, inducing expression of XBP1, a key mediator of the unfolded protein response (UPR) that allows expansion of the endoplasmic reticulum necessary for massive immunoglobulin production. In the context of FPP, this cascade appears constitutively activated in plasma cells resident in the pad dermis, as suggested by the intense immunostaining of MUM1/IRF4 and CD138 (syndecan-1) observed on biopsies (Mauldin 2016; Gross 2005).
The IL-6/JAK1/STAT3 signalling pathway plays a central role in plasma cell expansion. Interleukin 6, produced locally by macrophages and dermal fibroblasts in response to inflammatory signals, binds to its membrane receptor (IL-6Rα/gp130), activating the JAK1 tyrosine kinase which phosphorylates the STAT3 factor. Phosphorylated STAT3 dimerises and translocates to the nucleus, where it directly induces transcription of PRDM1 and IRF4 (Nutt 2015). This autocrine and paracrine circuit could be perpetuated in the pads by a microenvironment rich in pro-inflammatory cytokines, notably TNF-α and IL-1β, which in turn stimulate IL-6 production by stromal cells via activation of the canonical NF-κB (p65/RelA) pathway. The cytokine BAFF (B-cell Activating Factor, also called BLyS), a member of the TNF superfamily, constitutes a determining survival factor for long-lived plasma cells. BAFF binds to BCMA and TACI receptors expressed on the plasma cell surface, activating the non-canonical NF-κB pathway (RelB/p52) and inhibiting mitochondrial apoptosis through overexpression of BCL-2 and MCL-1 (Mackay 2009). Excess serum BAFF, documented in several human autoimmune diseases, could constitute an analogous pathophysiological mechanism in FPP, although measurement of this cytokine has not yet been performed specifically in affected cats.
4.2. Hypergammaglobulinaemia and immune complex deposits
Polyclonal hypergammaglobulinaemia, found in 50 to 63% of cats with FPP, reflects diffuse and non-specific activation of the plasma cell compartment (Guaguère 2004). Serum protein electrophoresis reveals elevation of the gamma fraction without restricted peak, with IgG levels that can reach two to three times the upper limit of normal. Excessive immunoglobulin production, in the absence of an identified target antigen, leads to the formation of circulating immune complexes that deposit in highly vascularised tissues, particularly renal glomeruli and pad dermis. These deposits have been demonstrated by direct immunofluorescence as granular deposits of IgG, IgM and C3 complement fraction along the epidermal basement membrane and around dermal vascular walls (Gross 2005). The deposition mechanism recalls that of type III hypersensitivity reaction (Gell and Coombs classification), where intermediate-sized immune complexes escape clearance by the reticulo-endothelial system and precipitate in vascular walls, triggering a local inflammatory cascade.
4.3. Complement activation and inflammatory cascade
Immune complex deposits activate the classical complement pathway through fixation of the C1q fragment to the Fc portion of aggregated immunoglobulins. This sequential activation (C1q → C1r/C1s → C4 → C2 → C3 convertase) leads to cleavage of C3 into C3a (anaphylatoxin) and C3b (opsonin), then to formation of the C5b-9 membrane attack complex which causes direct cell lysis (Gross 2005). The anaphylatoxins C3a and C5a act as powerful chemoattractants for neutrophils and macrophages, amplifying local inflammation. Release of lysosomal proteases (elastase, cathepsin G) by recruited neutrophils contributes to degradation of the pad dermis extracellular matrix, explaining the soft and spongy texture characteristic of affected tissue (Miller 2013). Measurement of serum C3, when performed, sometimes shows a decrease compatible with consumption by in vivo activation, a classic phenomenon of immune complex diseases.
4.4. Matrix metalloproteinases and tissue destruction
Destructive remodelling of pad connective tissue involves matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases capable of degrading extracellular matrix components. MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are particularly involved in the degradation of type IV collagen and laminin at the basement membrane level, whilst MMP-1 (interstitial collagenase) cleaves fibrillar collagen of types I and III which constitutes most of the pad dermal framework (Sapadin 2006). Expression of these MMPs is induced by TNF-α and IL-1β via activation of the AP-1 transcription factor (c-Fos/c-Jun) and the NF-κB pathway. Progressive collagen destruction, combined with inflammatory oedema and massive cellular infiltration, leads to the characteristic volume increase of the pad, whose consistency changes from firm to doughy. The central role of MMPs in pathogenesis provides a direct pharmacological rationale for the use of doxycycline, whose inhibitory activity on MMPs constitutes one of the best documented mechanisms of action independent of its antibacterial activity (Griffin 2010).
Clinical presentation
5.1. Footpad semiology
The clinical picture of FPP is usually characteristic and allows diagnostic orientation from macroscopic examination. The elementary lesion consists of diffuse and symmetrical swelling of one or more pads, giving the affected pad a tumescent, rounded appearance, noticeably larger than normal. The central metacarpal and metatarsal pads are affected preferentially in more than 80% of cases, although digital pads may also be involved (Miller 2013). The involvement is most often bilateral and simultaneously affects all four limbs in approximately 50% of reported cases (Guaguère 2004). The pad surface presents a bluish-purple to lilac colouration, sometimes described as local cyanosis, which reflects vascular engorgement and deep dermal inflammation. This tint is observed especially on lightly pigmented pads and may be masked on naturally dark pads. A highly evocative semiological sign resides in the striated appearance of the surface, with fine whitish crisscrossing striations forming a network (cross-hatching), which results from tension exerted by the oedematous tissue on the thinned pad epidermis (Gross 2005). Palpation reveals a soft and doughy consistency, clearly distinct from the normal firmness of a healthy pad, reflecting destruction of the dermal collagen architecture and massive cellular infiltration. Pain is generally absent in early stages, and lameness, when present, most often reflects secondary ulceration.
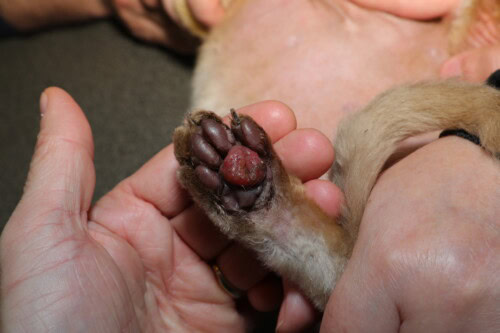
Advanced form with opening of the central pad
5.2. Natural evolution and complications
The evolution of FPP is variable and unpredictable. A significant proportion of cases, estimated between 10 and 30%, may present spontaneous resolution without therapeutic intervention, within a period of a few weeks to a few months (Miller 2013). This spontaneous regression reinforces the hypothesis of a reactional process to a transient antigenic stimulus. However, progression towards ulceration constitutes the most feared complication, occurring in 30 to 50% of untreated cases (Guaguère 2004). Ulceration manifests as a loss of substance of the pad epidermis, exposing the infiltrated and fragile dermis, often accompanied by secondary haemorrhage sometimes profuse due to inflammatory neovascularisation and fragility of vascular walls weakened by immune complex deposits and MMP action (Taylor 1990). Secondary bacterial superinfection of the ulcer constitutes an additional risk, although it is not systematic. Pain accompanying ulceration then causes marked lameness, reluctance to move and sometimes compulsive licking which perpetuates the erosive phenomenon. A few exceptional cases of recurrent bleeding having led to iron-deficiency anaemia have been documented, emphasising the need for haematological monitoring in cats presenting chronic ulcerations (Taylor 1990).
5.3. Associated extra-paw manifestations
FPP is not always limited to isolated paw involvement. Associations with other plasma cell manifestations have been documented, suggesting an underlying systemic process. Plasma cell stomatitis, characterised by plasma cell infiltration of the oral mucosa (particularly the palate and glossopalatine arches), has been described concomitantly with FPP in 10 to 20% of cases according to series (Miller 2013; Guaguère 2004). The simultaneous association of these two entities in the same animal constitutes a strong argument in favour of a systemic B compartment disorder. Renal involvement, in the form of immune complex glomerulonephritis or, more rarely, reactive type AA renal amyloidosis, has been reported in cats with chronic FPP (Dias Pereira 2003). Deposition of amyloid substance, consisting of fibrils derived from serum amyloid A protein (SAA) produced in excess by the liver under stimulation by IL-6 and TNF-α during chronic inflammation, can lead to progressive renal failure. These morbid associations confer on FPP a systemic dimension that exceeds the strictly dermatological framework and justifies a complete biological work-up in any diagnosed cat.
Diagnostic approach
6.1. Clinical examination and orientation criteria
Diagnosis of FPP rests on a bundle of clinical, cytological and histopathological arguments. Careful clinical examination of all four limbs, including inspection and palpation of all pads, constitutes the first step. The conjunction of painless swelling of one or more central pads, a purplish tint and doughy consistency in an adult cat strongly suggests the diagnosis. Complete dermatological examination must search for possible associated skin lesions at other sites, as well as examination of the oral cavity seeking concomitant plasma cell stomatitis. Evaluation of general condition, renal palpation and lymph node status form part of the initial clinical work-up (Miller 2013).
6.2. Contribution of cytology
Fine needle aspiration of the swollen pad, performed with a 22 to 25 gauge needle, constitutes a rapid and minimally invasive complementary examination, practicable during consultation. Slide preparation and May-Grünwald-Giemsa (MGG) or Diff-Quick staining reveals a cellular infiltrate composed of more than 80% mature plasma cells, recognisable by their eccentric nucleus, their “clock-face” chromatin and their abundant basophilic cytoplasm with a clear perinuclear halo corresponding to the hypertrophied Golgi apparatus (Gross 2005). The presence of Mott cells, plasma cells whose cytoplasm is distended by multiple spherical eosinophilic inclusions corresponding to Russell bodies (immunoglobulins aggregated in dilated endoplasmic reticulum), is a highly evocative sign of FPP. These inclusions result from dysfunction of the UPR pathway (XBP1-dependent) no longer able to manage the overload of secretory proteins (Nutt 2015). Cytology, although strongly suggesting the diagnosis, does not alone allow exclusion of extramedullary plasmacytoma, and histopathological confirmation remains recommended in atypical or unilateral cases.
6.3. Histopathology: the diagnostic standard
Skin biopsy, sampled with a scalpel wedge biopsy or biopsy punch (6 mm punch), provides definitive diagnosis. Histopathological examination reveals a diffuse or nodular dermal infiltrate composed almost exclusively of mature plasma cells, occupying the superficial and deep dermis, often extended to subcutaneous tissue (Gross 2005; Mauldin 2016). Plasma cells are well differentiated, without significant cytonuclear atypia, and the mitotic index is low, generally less than 1 mitosis per high-power field (×400), which formally distinguishes FPP from a plasmacytoma. The lobular architecture of pad adipose tissue is respected but invaded by the infiltrate. The epidermal basement membrane often appears thinned, and the overlying epidermis is atrophic, which predisposes to ulceration. Leukocytoclastic vasculitis, with fibrinoid necrosis of the dermal arteriole wall and parietal infiltration by neutrophils with fragmented nuclei, frequently accompanies the plasma cell infiltrate and testifies to the participation of immune complexes in pathogenesis (Gross 2005). Immunohistochemistry confirms expression of CD79a (pan-B marker), CD138 and MUM1/IRF4 by plasma cells, with polyclonal expression of kappa and lambda light chains excluding monoclonal proliferation (Mauldin 2016). Russell bodies are demonstrated by PAS (Periodic Acid-Schiff) staining, appearing as PAS-positive and diastase-resistant intracytoplasmic inclusions.
6.4. Biological and serological work-up
The biological work-up completes the diagnostic picture and provides prognostic information. The haemogram may reveal moderate lymphocytosis in some cats, as well as regenerative anaemia in case of chronic haemorrhages from ulcerated pads. Serum protein electrophoresis constitutes a key examination, demonstrating polyclonal hypergammaglobulinaemia in 50 to 63% of cases, with total protein levels sometimes exceeding 90 g/L (Guaguère 2004). Specific immunoglobulin assay, when available, shows predominantly elevated IgG, but IgA and IgM may also be increased. FIV and FeLV serology is systematically recommended, performed by rapid immunochromatography (detection of anti-FIV antibodies and FeLV p27 antigen) or by ELISA, with PCR confirmation in case of clinical discordance (Hartmann 2012). Renal biochemistry (creatinine, SDMA, urinary protein/creatinine ratio) must be evaluated to detect possible immune complex glomerulonephritis or associated renal amyloidosis. ANA testing by indirect immunofluorescence on HEp-2 cells completes the immunological work-up, although its positive predictive value remains modest in cats (Dias Pereira 2003).
6.5. Differential diagnosis
The differential diagnosis of FPP includes several entities affecting cat pads. Extramedullary plasmacytoma, rare in paw location, is distinguished by its generally solitary, unilateral character, and by a higher mitotic index (> 5 mitoses/10 fields ×400) associated with monoclonality on immunohistochemistry (Mauldin 2016). Eosinophilic granuloma that can affect pads is characterised by a predominantly eosinophilic infiltrate with flame collagenolysis images. Bacterial pododermatitis presents signs of infection (purulent exudate, hot swelling, marked pain). Pemphigus foliaceus, a frequent cause of crusting pododermatitis in cats, is distinguished by the presence of pustules, crusts and acantholysis on cytology and histology. Squamous cell carcinomas of the pads, observed especially in white cats, typically present with asymmetric ulcerative-proliferative lesions. Diffuse cutaneous mastocytosis, although very rare, can mimic pad swelling (Miller 2013; Hnilica 2017).
Therapeutic management
7.1. Therapeutic abstention and spontaneous resolution
The possibility of spontaneous resolution, documented in 10 to 30% of cases, constitutes a fundamental decision-making element (Miller 2013). In cats presenting limited, non-ulcerated and painless involvement, a period of observation of four to six weeks may be considered before any pharmacological intervention, with close clinical monitoring. This “watchful waiting” approach is all the more justified since available treatments are not without side effects and the disease does not threaten the vital prognosis in the absence of complications. The decision to treat rests on the presence of ulceration, pain, lameness, recurrent haemorrhages or progression of lesions despite surveillance (Bettenay 2003).
7.2. Doxycycline: pharmacological rationale and efficacy data
Doxycycline constitutes, since the pioneering work of Bettenay and collaborators, the most widely documented first-line treatment in the management of feline plasma cell pododermatitis. Its use does not rest on an antibacterial action — the condition having no demonstrated bacterial infectious aetiology — but on its pleiotropic immunomodulatory properties, shared with the entire tetracycline family (Bettenay 2003). On the molecular level, doxycycline inhibits the activity of several matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, by chelation of zinc ions necessary for their catalytic site. This inhibition limits degradation of type IV collagen and the basement membrane, processes directly implicated in the destructive tissue remodelling observed within affected pads (Guaguère 2004). Moreover, doxycycline exerts an anti-inflammatory action by reducing production of prostaglandin E2 (PGE2) via suppression of cyclo-oxygenase 2 (COX-2) expression in activated macrophages, and by decreasing nitric oxide (NO) synthesis through inhibition of inducible NO synthase (iNOS) (Sapadin 2006).
An additional mechanism, often underestimated in veterinary literature, concerns doxycycline’s capacity to modulate T lymphocyte activation. In vitro work demonstrates that tetracyclines reduce proliferation of mitogen-stimulated T lymphocytes and decrease expression of major histocompatibility complex class II (MHC-II) on the surface of antigen-presenting cells, thus attenuating the adaptive immune amplification loop (Niimi 1998). In the context of plasma cell pododermatitis, where polyclonal hypergammaglobulinaemia reflects dysregulated B activation, this action on antigen presentation holds direct pathophysiological relevance.
The standard therapeutic protocol rests on oral administration of doxycycline at a dosage of 10 mg/kg once daily, for a minimum duration of six to eight weeks (Bettenay 2003). Compliance may be compromised by the risk of oesophageal stricture, a cat-specific complication, induced by tablet retention in the oesophagus and local release of acidic pH causing focal mucosal necrosis. The prevalence of this complication has been estimated at approximately 3 to 5% of cats receiving solid forms of doxycycline without water monitoring (German 2005). Systematic administration of a 3 to 6 mL water bolus after each dose, or recourse to a liquid formulation, considerably reduces this risk. Retrospective data compiled by Guaguère and Bensignor report a complete or partial clinical response rate of 50 to 65% after a first cycle of doxycycline, with observable improvement from the third week of treatment in responders (Guaguère 2004). Resolution manifests as progressive decrease in paw swelling, repigmentation of pads and regression of characteristic whitish striations.
Relapses after treatment cessation constitute a frequent clinical reality, documented in approximately 30 to 50% of initially responding cases within three to six months (Scarampella 2018). This observation suggests that doxycycline does not correct the underlying pathogenic mechanism but attenuates its effector manifestations. The question of maintenance treatment at reduced dose (5 mg/kg/day or every other day) remains debated in the absence of a controlled trial, although some clinicians resort to it empirically with results deemed satisfactory in chronic recurrent forms.
7.3. Systemic glucocorticoids
Systemic glucocorticoids represent the second most frequently employed therapeutic line, particularly when doxycycline proves insufficient or when clinical severity requires rapid immunosuppression. Their mechanism of action rests on binding to the cytoplasmic glucocorticoid receptor (GR), which, after nuclear translocation, acts as a transcription factor modulating expression of more than 200 genes involved in immune and inflammatory response (Cain 2017). The main anti-inflammatory effect proceeds through inhibition of the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), thus blocking transcription of interleukin-1β (IL-1β), IL-6, TNF-α and multiple chemokines responsible for plasma cell recruitment within paw dermis.
Prednisolone, administered orally at a dose of 1 to 2 mg/kg/day for two to four weeks then in progressive decrease over four to six weeks, produces a clinical response in 60 to 70% of cases according to published retrospective series (Taylor 2004). Methylprednisolone acetate by subcutaneous injection (4 mg/kg) has been used in refractory cases, with reported efficacy but a less favourable long-term tolerance profile due to prolonged duration of action and impossibility of dosage adjustment (Guaguère 2004). Dose-dependent adverse effects — polyuria-polydipsia, polyphagia, increased susceptibility to diabetes mellitus, urinary tract infections — limit long-term use in cats, a species notoriously sensitive to corticoid-induced insulin resistance. Development of iatrogenic diabetes mellitus has been estimated at 5 to 10% in cats receiving glucocorticoids for more than three months, this prevalence varying according to cumulative dose and individual risk factors such as obesity and advanced age (Lowe 2008).
Glucocorticoid weaning constitutes a delicate therapeutic time. Excessively rapid decrease exposes to relapse, whilst prolonged maintenance increases iatrogenic risks. Initial doxycycline-prednisolone combination, with relay by doxycycline alone after obtaining clinical remission, offers a pragmatic compromise often adopted in specialist practice (Scarampella 2018).
7.4. Ciclosporin and calcineurin inhibitors
Ciclosporin (ciclosporin A, CsA) has emerged as a therapeutic alternative of choice in refractory forms of feline plasma cell pododermatitis. Its mechanism of action, distinct from that of glucocorticoids, rests on formation of a complex with intracellular cyclophilin; this complex inhibits calcineurin, a calcium-dependent phosphatase essential for dephosphorylation of the NFAT (Nuclear Factor of Activated T-cells) transcription factor. Blockage of NFAT prevents transcription of IL-2, a major cytokine of T helper lymphocyte clonal proliferation, and consequently reduces T help provided to B lymphocytes for isotype switching and plasma cell differentiation (Robson 2003). In a disease characterised by dense plasma cell infiltration and hypergammaglobulinaemia, this targeting of T-B dialogue holds direct pathophysiological relevance.
The recommended dosage in cats is 5 to 7 mg/kg/day orally, on an empty stomach to optimise bioavailability, which varies from 25 to 35% according to individuals (Latimer 2014). Several case series report response rates of 60 to 85% with ciclosporin, including in animals not having responded to doxycycline or glucocorticoids (Bettenay 2003; Scarampella 2018). The onset of action is generally four to six weeks, a delay consistent with the mode of action on adaptive immune response. Digestive adverse effects — vomiting, diarrhoea — affect approximately 15 to 25% of cats at treatment initiation, but frequently resolve through progressive introduction of the dose over one week or by concomitant administration with a small amount of food (Roberts 2016).
Opportunistic infection risk merits careful monitoring. Toxoplasmosis reactivation, although rarely documented under ciclosporin in cats, has been reported during prolonged immunosuppression and constitutes a relative contraindication in seronegative individuals exposed to a risk environment (Last 2004). Pre-therapeutic Toxoplasma gondii serological work-up is recommended by several experts, although the level of evidence remains limited for this specific indication. Topical tacrolimus (FK-506), another calcineurin inhibitor, has not been the subject of targeted studies in plasma cell pododermatitis, probably due to the difficulty of application on the irregular pad surface and poor percutaneous penetration through thickened paw epidermis.
7.5. Other immunomodulators
Several immunomodulatory agents have been employed occasionally, without any benefiting from a level of evidence equivalent to that of doxycycline or ciclosporin. Chlorambucil, an alkylating agent of the nitrogen mustard family, has been used in severe forms recalcitrant to therapy at a dosage of 0.1 to 0.2 mg/kg every other day, in combination with prednisolone (Taylor 2004). Its mechanism rests on alkylation of lymphocyte DNA in proliferation, inducing interstrand linkages that prevent replication and transcription, with preferential action on B lymphocyte lineages, which theoretically justifies its use in a plasma cell-mediated disease. The haematological toxicity profile requires monitoring by complete blood count every two to three weeks during the first two months of treatment. Significant myelosuppression, defined by neutropenia below 2,500/μL, occurs in approximately 10 to 15% of treated cats and requires temporary suspension (Lowe 2008).
Mycophenolate mofetil, a selective and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), a key enzyme of de novo purine synthesis in lymphocytes, has been reported anecdotally in a few cases of plasma cell pododermatitis with variable results, at a dosage of 10 mg/kg twice daily orally (Backel 2013). The absence of a controlled trial and scarcity of pharmacokinetic data in cats limit its recommendation.
Recombinant interferons represent a distinct immunomodulatory approach. Recombinant feline omega interferon (rFeIFN-ω), whose action proceeds through activation of the JAK-STAT pathway (particularly STAT1 and STAT2) via the type I interferon receptor, induces expression of interferon-stimulated genes (ISGs) and modulates the Th1/Th2 balance, which could theoretically correct the immune bias underlying plasma cell infiltration (de Mari 2004). A few clinical cases have reported improvement after subcutaneous or per-mucosal injection of omega interferon, but data remain too sporadic to draw solid conclusions.
7.6. Surgical treatment
Surgical excision of affected pads constitutes an option reserved for focal forms severely ulcerated with secondary septic complications or for cases totally refractory to all medical options. Podoplasty has been described in isolated case reports, with resection of severely remodelled tissue followed by closure by advancement flap or second-intention healing under semi-occlusive dressing (Guaguère 2004). Functional results depend on the extent of resection and number of pads involved. Multi-paw involvement, which concerns 40 to 50% of cases, makes this approach technically difficult and ethically questionable when it would involve bilateral resection of weight-bearing pads.
Digital amputation has been reported in rare situations where a single digital pad presented irreducible swelling with deep ulceration and secondary osteomyelitis, but this eventuality remains exceptional in plasma cell pododermatitis, which rarely reaches the underlying bone (Taylor 2004). Recourse to CO₂ laser for controlled vaporisation of excess tissue has been mentioned empirically, but no study formally evaluates its efficacy or healing profile in this specific indication. Post-operative management systematically involves concomitant immunomodulatory treatment to prevent local recurrence, confirming that surgery treats only the tissue consequence without correcting the causal immune dysregulation.
Prognosis and long-term monitoring
8.1. Response rates and remission duration
Prognostic evaluation of feline plasma cell pododermatitis rests on essentially retrospective data, the absence of randomised controlled trials constituting a major limitation in comparative assessment of therapeutic response rates. Published series, collectively grouping between 100 and 150 documented cases over the past three decades, converge towards a globally favourable prognostic profile for non-ulcerated forms managed early (Guaguère 2004; Scarampella 2018). The complete remission rate, defined as total clinical resolution of paw lesions and normalisation of pad texture, varies from 30 to 50% according to publications and treatment employed. Partial remission, characterised by clinical improvement exceeding 50% but with persistence of residual thickening or depigmentation, concerns an additional 20 to 30% of treated cases.
The natural history of the disease is remarkable for the possibility of spontaneous remissions, reported in 10 to 20% of cases according to the most complete retrospective series published to date (Bettenay 2003). This phenomenon, exceptional in feline immune-mediated diseases, raises the question of an endogenous immunological regulation capable of restoring tolerance or suppressing the pathogenic plasma cell clone. The hypothesis of modulation by regulatory T lymphocytes (Treg), via production of IL-10 and TGF-β, which exert negative feedback on B differentiation and plasma cell survival, could explain this auto-resolution phenomenon (Groux 1997). Direct demonstration of this hypothesis in plasma cell pododermatitis remains to be established through dynamic immunophenotyping studies.
Remission duration after medical treatment varies considerably. In doxycycline responders, median remission duration has been estimated between three and twelve months, with a bimodal distribution: a group of durable responders (remission exceeding one year) and a group of early relapsers (remission less than three months) (Scarampella 2018). This heterogeneity suggests the existence of pathogenically distinct sub-populations, potentially identifiable by immunological or histopathological biomarkers that remain to be defined. Under ciclosporin, prolonged remissions appear more frequent, with reported median durations of six to eighteen months, although therapeutic dependence — need to maintain low-dose maintenance treatment — concerns a significant proportion of cases (Bettenay 2003).
8.2. Identified prognostic factors
Identification of reliable prognostic factors remains a challenge in the absence of sufficiently large prospective cohorts. Several clinical and biological parameters have been associated, retrospectively and with variable levels of significance, with the probability of therapeutic response. The degree of ulceration at diagnosis constitutes the most regularly identified negative prognostic factor: pads presenting deep ulceration with exposure of underlying dermis respond less favourably to doxycycline alone and more frequently require recourse to second-line immunosuppression (Taylor 2004). The pathophysiological explanation resides in irreversible fibrous remodelling and secondary bacterial colonisation which perpetuate the local inflammatory cycle independently of the primary immunological mechanism.
The level of serum gammaglobulins at diagnosis appears inversely correlated with prognosis: cats presenting marked hypergammaglobulinaemia (exceeding 20 g/L) display lower complete remission rates than those whose gammaglobulins remain moderately elevated (Gruffydd-Jones 1980; Guaguère 2004). This observation reinforces the idea of a severity continuum reflected by the amplitude of systemic immune activation. The number of pads affected at initial presentation has also been correlated with prognosis: involvement of four or more pads is associated with a significantly higher probability of relapse than that observed in single-paw or two-paw forms (Scarampella 2018). FIV serology, when positive, does not appear to influence therapeutic response in a determining manner in available series, although the number of co-infected cases remains too small to draw statistically robust conclusions (Bettenay 2003).
Age at diagnosis has not been identified as an independent prognostic factor in a reproducible manner, although some authors report a tendency towards more severe and more recurrent forms in individuals under three years, potentially linked to immaturity of immunological tolerance mechanisms (Taylor 2004). Development of standardised clinical scores, integrating lesional extent, degree of ulceration, biological parameters and infiltrating immunophenotype, constitutes an unmet need that would allow homogenisation of prognostic evaluation and comparison between studies.
Comorbidities and concept of feline plasma cell syndrome
9.1. Plasma cell stomatitis
The association between feline plasma cell pododermatitis and plasma cell stomatitis (also designated chronic feline gingivostomatitis with plasma cell predominance) has been recognised since the princeps descriptions of the disease and constitutes the strongest clinical argument in favour of a systemic plasma cell syndrome in cats (Gruffydd-Jones 1980). The prevalence of co-occurrence varies from 10 to 35% according to series, the higher range being reported in studies that systematically explored the oral cavity in cats with pododermatitis (Guaguère 2004; Scarampella 2018). Plasma cell stomatitis is characterised histologically by dense plasma cell infiltrate of the gingival and buccal lamina propria, with Mott cells and Russell bodies identical to those observed in pads. Immunohistochemical analysis reveals a predominance of polyclonal IgG, similar to the serum profile, and local overexpression of IL-1β, IL-6 and TNF-α in affected mucosae (Harley 2003).
On the molecular level, chronic feline stomatitis has been associated with activation of the NF-κB pathway and overexpression of RANKL (Receptor Activator of Nuclear Factor Kappa-B Ligand) in gingival tissues, contributing to alveolar bone resorption observed in advanced forms (Arzi 2010). This latter pathway distinguishes stomatitis from pododermatitis, where the osteolytic component is absent or minimal. Stomatitis-pododermatitis comorbidity does not appear linked to FIV or FeLV status exclusively, since it is documented in cats seronegative for these two retroviruses (Bettenay 2003). This finding reinforces the hypothesis of a common immunological background predisposing to aberrant plasma cell activation in distinct anatomical sites sharing antigenic exposure characteristics (oral mucosae and paw epithelium, both subjected to chronic mechanical and microbial stimulations).
9.2. Glomerulonephritis and renal amyloidosis
Renal involvement constitutes a potentially serious complication of feline plasma cell pododermatitis, reported in a limited but significant number of cases. Immune complex glomerulonephritis has been histologically documented in cats presenting plasma cell pododermatitis associated with proteinuria and progressive azotaemia (Gruffydd-Jones 1980). The pathogenic mechanism involves deposition of circulating immune complexes — formed by excess polyclonal immunoglobulins and their corresponding antigens — in the glomerular mesangium and along the glomerular basement membrane, with local complement activation by the classical pathway (C1q → C4 → C2 → C3) and recruitment of neutrophils and macrophages contributing to endothelial and podocyte lesions (Center 1990).
Type AA renal amyloidosis has been reported in rare cases of chronic plasma cell pododermatitis, serum amyloid A protein (SAA) being an acute phase protein synthesised by hepatocytes under the effect of IL-6 and IL-1β — cytokines whose production is chronically elevated in systemic plasma cell inflammation (DiBartola 1997). The exact prevalence of renal involvement remains difficult to estimate in the absence of systematic evaluation of proteinuria and renal function in published cohorts. However, recommendation of a urinary protein/creatinine ratio (UPCR) at diagnosis and in longitudinal monitoring appears justified in cats presenting marked hypergammaglobulinaemia or chronic disease evolving for more than six months (Scarampella 2018).
9.3. Towards a unified nosological spectrum
Accumulation of clinical and histopathological observations leads to considering plasma cell pododermatitis not as an isolated dermatological entity, but as the paw manifestation of a systemic feline plasma cell syndrome. This concept, implicit in the work of Gruffydd-Jones since 1980, has been formalised by Guaguère and Bensignor, who propose a nosological framework integrating pododermatitis, plasma cell stomatitis, immune complex glomerulonephritis and, more rarely, extra-paw plasma cell dermatitis affecting the pinna or nasal planum (Guaguère 2004). The pathophysiological unity of this spectrum rests on the common denominator of dysregulation of terminal B-plasma cell differentiation, with excessive polyclonal immunoglobulin production and tissue deposition of plasma cells in anatomical sites predisposed by their local immune microenvironment.
This model recalls, by analogy, monoclonal gammopathies of undetermined significance (MGUS) and low-grade lymphoproliferative syndromes in human medicine, without being able to establish a strict parallel due to the polyclonal — and not monoclonal — character of B activation observed in feline plasma cell syndrome (Mellor 2006). The boundary between reactive polyclonal activation and pre-neoplastic clonal proliferation would merit exploration by B clonality techniques (PCR-PARR, for PCR for Antigen Receptor Rearrangement), which would allow detection of a possible dominant subclone within the apparently polyclonal infiltrate. Werner and collaborators’ study, having applied the PARR technique to plasma cell pododermatitis biopsies, confirmed the polyclonal character in the majority of tested cases, but oligoclonal rearrangement was identified in approximately 15% of samples, opening the question of possible progression towards MALT (Mucosa-Associated Lymphoid Tissue) type lymphoma in certain chronic cases (Werner 2011).
Perspectives and research directions
10.1. Diagnostic and prognostic biomarkers
Development of objective, non-invasive or minimally invasive biomarkers represents a priority for improving early diagnosis, therapeutic monitoring and prognostic stratification of feline plasma cell pododermatitis. Serum protein electrophoresis, although useful for documenting hypergammaglobulinaemia, lacks specificity and sensitivity for longitudinal monitoring. Assay of serum free light chains (sFLC), widely used in human haematology for monitoring monoclonal gammopathies and myelomas, constitutes a relevant candidate biomarker. Free kappa and lambda light chains are produced in excess during plasma cell activation and their κ/λ ratio reflects the clonal balance or imbalance of the B population (Bradwell 2001). An ELISA test adapted to feline immunoglobulins has been developed, but its analytical and clinical validation in the specific context of plasma cell pododermatitis remains to be performed (Tasca 2018).
Serum cytokines offer another biomarker exploration axis. Assay of IL-6, whose role in plasma cell survival via STAT3 activation is well established in human oncology (Kishimoto 2005), could allow identification of patients at risk of systemic forms (glomerulonephritis, amyloidosis). Similarly, serum BAFF (B-cell Activating Factor of the TNF Family), a major cytokine of mature B lymphocyte and plasma cell survival via activation of the non-canonical NF-κB pathway (RelB/p52), constitutes a potential biomarker and therapeutic target. Elevated BAFF levels have been correlated with severity of systemic autoimmune diseases in humans (systemic lupus erythematosus, Sjögren’s syndrome), and extrapolation to feline plasma cell syndrome appears biologically founded, although not yet experimentally explored (Mackay 2009).
The advent of tissue transcriptomics by RNA sequencing (RNA-seq) applied to pad biopsies would allow mapping of gene expression in the plasma cell infiltrate, identification of molecular signatures associated with therapeutic response and potentially discovery of hitherto unsuspected therapeutic targets. The decreasing cost of next-generation sequencing makes this approach feasible in the framework of multicentre studies, provided a tissue biobank is constituted in a coordinated manner.
10.2. Emerging targeted therapies
Beyond JAK inhibitors already mentioned, several emerging therapeutic classes in human medicine could find application in feline plasma cell syndrome. Anti-BAFF antibodies (belimumab) and anti-APRIL (A PRoliferation-Inducing Ligand, a BAFF-related ligand), which block plasma cell survival signals, have demonstrated efficacy in human lupus with significant reduction in autoantibody titres and disease activity decrease (Furie 2011). Development of feline-specific monoclonal anti-BAFF antibodies would represent a considerable advance, but encounters the usual obstacles of development cost, GMP (Good Manufacturing Practice) production and regulated veterinary clinical trials.
Proteasome inhibitors, such as bortezomib, which induce plasma cell apoptosis through accumulation of misfolded proteins and activation of the endoplasmic reticulum stress pathway (UPR, Unfolded Protein Response), constitute an attractive approach for selectively targeting disease effector cells (Neubert 2008). Their use in veterinary medicine remains confined to experimental oncology, with limited toxicological data in cats (thrombocytopenia, potential peripheral neurotoxicity).
Autologous regulatory T cell-based therapies expanded ex vivo, which aim to restore immunological tolerance through adoptive transfer of CD4+CD25+FoxP3+ Treg, represent the most ambitious horizon of research in immunotherapy of autoimmune diseases. Cell sorting, expansion and reinjection protocols developed in humans (Bluestone 2015) could theoretically be adapted to cats, but technical and financial challenges remain considerable, and no preclinical trial has been initiated in the specific context of plasma cell pododermatitis.
Conclusion
Feline plasma cell pododermatitis, long considered an anecdotal dermatological curiosity, now stands out as a fascinating clinical model at the interface of veterinary dermatology, immunology and haematology. Understanding of this disease has progressed substantially since the princeps descriptions by Gruffydd-Jones in 1980, moving from a purely descriptive entity to an immunopathological syndrome whose molecular mechanisms — polyclonal plasma cell activation, dysregulation of IL-6/STAT3 and BAFF/NF-κB axes, immune complex deposition — are now partially elucidated. The therapeutic arsenal, dominated by doxycycline and ciclosporin, offers satisfactory response rates in the majority of cases, but the recurrent profile of the disease and heterogeneity of individual responses highlight the limits of current approaches. The concept of systemic feline plasma cell syndrome, integrating pododermatitis, plasma cell stomatitis and renal involvement, provides a coherent nosological framework that should guide future research towards systematic evaluation of extra-paw manifestations and prognostic stratification based on objective biomarkers. The rise of targeted therapies and regenerative medicine approaches offers encouraging prospects, provided the veterinary community equips itself with multicentre prospective studies and case registries allowing achievement of the statistical power necessary for validation of these innovations.
Bibliography
Arzi B, Murphy B, Cox DP, Vapniarsky N, Kass PH, Verstraete FJ. Presence and quantification of mast cells in the gingiva of cats with tooth resorption, periodontitis and chronic stomatitis. Arch Oral Biol. 2010;55:148-154.
Backel KA, Cain CL, Mauldin EA. Canine and feline plasma cell pododermatitis: retrospective analysis of clinical and histopathologic findings. Vet Dermatol. 2013;24:404.
Bettenay SV, Mueller RS, Dow K, Friend S. Prospective study of the treatment of feline plasmacytic pododermatitis with doxycycline. Vet Rec. 2003;152:564-566.
Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Helber MK. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189.
Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673-680.
Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233-247.
Center SA, Smith CA, Wilkinson E, Erb HN, Lewis RM. Clinicopathologic, renal immunofluorescent, and light microscopic features of glomerulonephritis in the dog. J Am Vet Med Assoc. 1990;190:81-90.
de Mari K, Maynard L, Eun HM, Lebreux B. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled field trial. Vet Rec. 2004;152:105-108.
DiBartola SP, Tarr MJ, Webb DM, Giger U. Familial renal amyloidosis in Chinese Shar-Pei dogs. J Am Vet Med Assoc. 1997;197:483-487.
Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D. A phase III, randomised, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918-3930.
German AJ, Cannon MJ, Dye C, Booth MJ, Pearson GR, Reay CA. Oesophageal strictures in cats associated with doxycycline therapy. J Feline Med Surg. 2005;7:33-41.
Gonzales AJ, Bowman JW, Fici GJ, Zhang M, Mann DW, Mitton-Fry M. Oclacitinib (APOQUEL) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther. 2014;37:317-324.
Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742.
Gruffydd-Jones TJ, Orr CM, Lucke VM. Foot pad swelling and ulceration in cats: a report of five cases. J Small Anim Pract. 1980;21:31-40.
Guaguère E, Bensignor E. Pododermatite plasmocytaire féline. Prat Méd Chir Anim Comp. 2004;39:29-36.
Harley R, Gruffydd-Jones TJ, Day MJ. Immunohistochemical characterisation of oral mucosal lesions in cats with chronic gingivostomatitis. J Comp Pathol. 2003;128:147-160.
Kishimoto T. Interleukin-6: from basic science to medicine — 40 years in immunology. Annu Rev Immunol. 2005;23:1-21.
Last RD, Suzuki Y, Manning T, Lindsay D, Galipeau L, Bhopale V. A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet Dermatol. 2004;15:194-198.
Latimer KS. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology. 5th ed. Wiley-Blackwell; 2014.
Lowe AD, Campbell KL, Graves TK. Glucocorticoids in the cat. Vet Dermatol. 2008;19:340-347.
Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491-502.
Mellor PJ, Haugland S, Smith KC, Powell RM, Gaskell RM, Knottenbelt CM, Argyle DJ. Histopathologic, immunohistochemical, and cytologic analysis of feline myeloma-related disorders: further evidence for primary extramedullary development in the cat. Vet Pathol. 2006;43:234-246.
Neubert K, Meister S, Moser K, Tolber C, Peddinghaus A, Nimmerjahn F, Schett G, Voll RE. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748-755.
Niimi A, Torigoe R, Sato M. Immunomodulatory effects of tetracyclines on human T-lymphocyte proliferative response. Clin Exp Immunol. 1998;111:328-332.
Ortalda C, Noli C, Colombo S, Borio S. Oclacitinib in feline nonflea-, nonfood-induced hypersensitivity dermatitis: results of a small prospective pilot study of client-owned cats. Vet Dermatol. 2015;26:235-e52.
Quimby JM, Webb TL, Habenicht LM, Dow SW. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48.
Roberts ES, Speranza C, Friberg C, Griffin C, Steffan J, Roycroft L, Alva R. Confirmatory field study for the effectiveness and safety of cyclosporine in the control of feline hypersensitivity dermatitis. J Vet Pharmacol Ther. 2016;39:487-493.
Robson D, Burton G. Cyclosporin: applications in small animal dermatology. Vet Dermatol. 2003;14:1-9.
Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265.
Scarampella F, Ordeix L. Doxycycline therapy in 10 cases of feline plasma cell pododermatitis: clinical, haematological and serological evaluations. Vet Dermatol. 2018;29:467-e158.
Tasca S, Carli E, Caldin M, Menegazzo L, Furlanello T, Solano-Gallego L. Serum free light chain concentrations in dogs and cats with various diseases. Vet Clin Pathol. 2018;47:419-427.
Taylor JE, Schmeitzel LP. Plasma cell pododermatitis with chronic footpad haemorrhage in two cats. J Am Vet Med Assoc. 2004;197:375-377.
Werner AH, Gruendl S, Geisweid K, Mueller RS. Clonality testing in feline lymphoma by PCR for antigen receptor rearrangement (PARR). Vet Immunol Immunopathol. 2011;143:181-186.
White SD, Rosychuk RAW, Janik TA, Denerolle P, Schultheiss P. Plasma cell stomatitis-pharyngitis in cats: 40 cases (1973-1991). J Am Vet Med Assoc. 2000;200:1377-1380.