Cutaneous calcinoses represent a major diagnostic and therapeutic challenge in canine veterinary dermatology. These conditions, characterized by abnormal accumulation of calcium salts in dermal and hypodermal structures, require a rigorous clinical approach based on precise understanding of their distinct pathophysiological mechanisms.
September 2025
Early identification of different types of calcinosis and implementation of appropriate treatment directly determine the functional and aesthetic prognosis. During the recent annual GEDAC conference, our colleague Vincent Bruet, president of GEDAC, presented recent advances in etiological classification, contemporary diagnostic modalities and emerging therapeutic perspectives, particularly those inspired by protocols developed in human medicine.
Definition and Nomenclature of Cutaneous Calcinosis
Cutaneous calcinosis is precisely defined by abnormal deposits of calcium salts in the dermis and hypodermis. These mineral accumulations manifest clinically as indurated nodules or plaques, typically presenting a characteristic whitish coloration, and are likely to significantly alter the cutaneous surface. Modern classification distinguishes three main clinical entities according to their anatomical extension and morphological presentation.
Cutaneous calcinosis constitutes the generic term encompassing all calcium deposits affecting cutaneous structures, regardless of their location or extent. This general denomination allows identification of the fundamental pathological process without prejudging its specific characteristics.
Calcinosis circumscripta represents a localized clinical form, characterized by circumscribed calcium deposits, frequently observed in peri-articular regions or near extremities. This preferential localization suggests the intervention of local mechanical factors in the genesis of these lesions.
Calcinosis universalis constitutes the most extensive and severe form, characterized by diffuse involvement affecting not only cutaneous structures but also subcutaneous, muscular and tendinous tissues. This systemic form is generally associated with a more reserved prognosis and requires complex multidisciplinary management.
Pathophysiological Foundations and Modern Classification of Calcinoses
The current taxonomy of cutaneous calcinoses is structured around five distinct pathophysiological entities, each responding to specific biological mechanisms. This stratification constitutes the foundation of any rational therapeutic approach, as it determines diagnostic orientation and conditions therapeutic choices.
Dystrophic calcinosis
It occupies a central position in veterinary dermatology. Its development occurs exclusively on tissues previously altered by inflammatory, necrotic or degenerative processes, in a physiological metabolic context characterized by normocalcemia and normophosphatemia. Initial tissue damage triggers a complex molecular cascade involving the release of cellular proteins with phosphatic affinity, notably mitochondrial proteins, which favor the formation of crystalline nucleation foci, then the progressive precipitation of calcium compounds around fibrillar structures of the dermis, particularly collagen and elastin fibers.
In human medicine, this form represents the most frequent manifestation and affects up to 70% of children with dermatomyositis. Connective tissue diseases constitute the main etiologies, including scleroderma, dermatomyositis, lupus erythematosus and mixed connective tissue diseases. Infectious processes, neoplastic conditions and certain hereditary dermatoses such as Ehlers-Danlos syndrome complete the etiological spectrum of this form.
Metastatic Calcinosis
It proceeds from a radically different mechanism, involving calcium precipitation in structurally intact tissues. This form results directly from systemic metabolic abnormalities causing hypercalcemia, hyperphosphatemia, or both simultaneously. Hyperparathyroidism, whether primary or secondary, constitutes the main triggering factor through three convergent metabolic pathways: activation of osteoclastic resorption, increased renal tubular reabsorption of calcium, and indirect stimulation of calcitriol synthesis which intensifies intestinal calcium absorption.
The concept of calcium-phosphate product is of paramount importance here. When this product (serum calcium × serum phosphorus) crosses the critical threshold of 70 mg²/dL², spontaneous crystallization phenomena begin in various organic structures. Although visceral organs (stomach, lungs, kidneys, myocardium) constitute the preferred targets, the skin can also be affected, although more rarely.
Particular and Emerging Forms
Idiopathic calcinosis represents an enigmatic entity characterized by the absence of any detectable metabolic abnormality and pre-existing tissue lesion. This form raises fundamental questions concerning the existence of still unknown pathophysiological mechanisms or latent genetic predispositions. In human medicine, this category includes tumoral calcinosis, subepidermal calcified nodules and scrotal calcinosis.
Iatrogenic calcinosis results directly from specific medical interventions or treatments. Local or systemic administration of products containing calcium or phosphate, such as calcium gluconate, can cause localized calcium deposits. Excessive vitamin D supplementation also constitutes a recognized risk factor, particularly in animals receiving uncontrolled nutritional supplements.
Calciphylaxis undoubtedly constitutes the most formidable form. This entity is distinguished by selective calcification of the media of dermal and hypodermal arterioles, generating a progressive ischemic process. The clinical consequences prove dramatic: extensive cutaneous necroses, tissue damage, recurrent septic superinfections and high mortality. This form remains closely associated with terminal stages of chronic renal failure and is particularly observed in dialyzed patients in human medicine.
Detailed Pathophysiological Mechanisms of Dystrophic Calcinosis
In dystrophic calcinosis, serum calcium and phosphate levels remain within normal physiological limits. The pathological process is initiated by tissue damage that leads to massive release of intracellular proteins, notably mitochondrial proteins endowed with particular affinity for phosphate. These proteins act as nucleation centers, favoring local precipitation of calcium salts.
Local tissue hypoxia, a direct consequence of initial lesions, creates an environment favorable to calcification. Chronic inflammation maintains this process through sustained release of pro-inflammatory cytokines, particularly TNF-α, IL-6 and IL-1β. These inflammatory mediators amplify the local tissue response and perpetuate conditions conducive to pathological mineralization.
The formation of intracellular crystals constitutes a major deleterious event, inevitably leading to cellular necrosis. This cell death releases new nucleation foci and maintains a self-sustaining vicious circle of progressive calcification. The process gradually extends to adjacent structures, particularly collagen and elastin fibers of the dermis, creating the characteristic lesions observed clinically.
Epidemiology and Etiological Particularities in Canine Veterinary Medicine
Etiological Distribution and Quantitative Data
Epidemiological analysis reveals a particularly characteristic etiological distribution in canine veterinary medicine. A large-scale retrospective study has precisely established the distribution of underlying causes, revealing patterns distinct from those observed in human medicine.
Hypercortisolism massively dominates the etiological landscape, representing approximately 80% of all observed cases. This predominance is explained by the frequency of systemic corticosteroid therapies in veterinary medicine and by the non-negligible incidence of spontaneous Cushing’s syndromes in the canine species. Within this majority category, iatrogenic hypercortisolism, a direct consequence of prolonged corticosteroid treatments, constitutes about 70% of cases, while endogenous hypercortisolism (pituitary and adrenal) represents the remaining 30%.
This predominance of iatrogenic hypercortisolism is explained by the frequent use of corticosteroids in veterinary medicine for the treatment of various inflammatory, allergic and autoimmune conditions. The easy access to these molecules and their immediate therapeutic efficacy sometimes favor their prolonged prescription, creating conditions conducive to the development of cutaneous calcinoses.
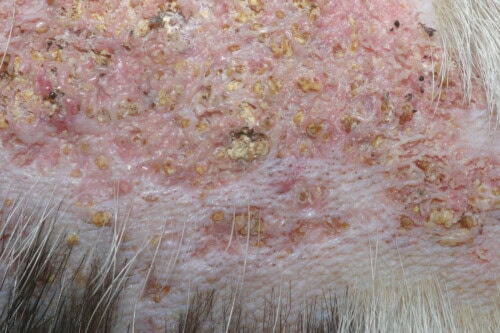
Cutaneous calcinosis: Close-up view
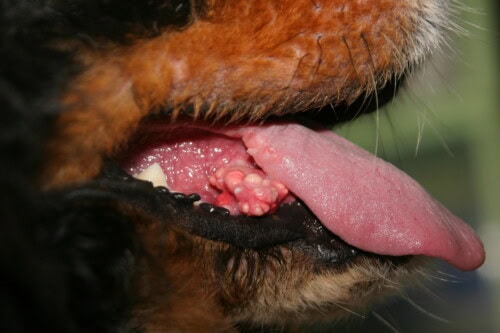
Cutaneous calcinosis: Sublingual lesions
Minority Causes and Particular Situations
Chronic renal failure occupies the second etiological position with approximately 10% of documented cases. This relatively modest proportion is explained by the predilection of metastatic calcium deposits for visceral soft tissues rather than cutaneous structures in this pathology. Nevertheless, cutaneous forms, when they occur, are generally associated with advanced stages of renal disease and constitute an unfavorable prognostic indicator.
The last decile groups a heterogeneous set of rare but clinically significant etiologies. Post-traumatic dystrophic calcinosis can occur following repeated trauma or chronic mechanical lesions, particularly in areas subjected to recurrent friction or sustained mechanical pressure.
Post-inflammatory forms are sometimes observed in the context of severe systemic infections, particularly deep mycoses such as blastomycosis or histoplasmosis, and certain leptospirosis. These infections create a systemic inflammatory state conducive to the development of dystrophic calcification lesions.
Idiopathic calcinosis, although rare, deserves particular attention as it raises fundamental questions about our understanding of pathophysiological mechanisms. The absence of an identified underlying cause suggests the existence of genetic or environmental predisposing factors still unknown.
Non-corticosteroid iatrogenic calcinoses, related to calcium solution infusions or excessive vitamin supplementation, complete this etiological picture. These preventable forms underline the importance of careful monitoring during administration of potentially calcifying treatments.
Racial Considerations and Predispositions
Although no racial exclusivity is formally established, certain clinical observations suggest a predisposition of large breeds. This tendency could be explained by increased susceptibility to endocrine pathologies, notably hyperadrenocorticism, or by differences in therapeutic practices according to animal size.
The English Bulldog is the subject of particular attention in veterinary literature, with certain data suggesting a specific predisposition in this breed. This susceptibility could be linked to the anatomical particularities of the breed, notably the numerous skin folds creating repeated microtrauma, or to specific genetic factors still not elucidated.
Brachycephalic breeds as a whole might present a relative predisposition, possibly in relation to their propensity for chronic respiratory disorders requiring prolonged anti-inflammatory treatments. This hypothesis, however, requires more thorough epidemiological studies to be confirmed.
Clinical Semiology and Contemporary Diagnostic Approach
Polymorphism of Clinical Manifestations
The clinical presentation of canine cutaneous calcinoses is characterized by remarkable polymorphism, reflecting the diversity of underlying pathophysiological mechanisms and the heterogeneity of individual tissue responses. This semiological variability sometimes constitutes a considerable diagnostic challenge, requiring a methodical and experienced clinical approach.
Anatomical localization classically favors the dorsal region, constituting the most frequently observed site of involvement. However, involvement can extend to glabrous ventral areas, particularly the abdomen and inguinal region, to skin folds where maceration phenomena favor local inflammation, to the cervical region and armpits. This non-random topographic distribution suggests the intervention of local mechanical factors, notably friction and repeated pressure, or regional vascular particularities in the development and progression of lesions.
Predilection zones often correspond to regions subjected to particular mechanical constraints or presenting specific anatomical characteristics. Skin folds, for example, create a humid and warm microenvironment conducive to chronic inflammation, while areas of contact with the ground or bedding surfaces undergo repeated low-intensity trauma.
Morphological Characteristics of Lesions
Lesional appearance presents two distinct main phenotypes, each probably corresponding to different evolutionary stages or specific pathophysiological mechanisms. The first phenotype manifests as firm to frankly hard plaques, displaying a characteristic yellowish coloration and circumscribed by a marked erythematous ridge testifying to the intense perilesional inflammatory reaction. This presentation generally corresponds to acute or subacute stages of the pathological process.
The second phenotype corresponds to extensive alopecic plaques, remarkably indurated, presenting an intense pinkish tint evocative of underlying chronic inflammation. These lesions generally testify to a more prolonged evolution and chronicization of the local inflammatory process.
Palpation reveals a characteristic consistency, oscillating between marked firmness and frankly stony hardness according to the degree of mineralization. This induration constitutes a pathognomonic semiological element, allowing differentiation of calcinoses from other chronic inflammatory dermatoses.
The lesional surface may present various alterations: localized hyperkeratosis, formation of superficial crusts, exudation of variable composition, and in the most evolved cases, frank ulceration with exposure of the underlying dermis. The presence of purulent exudate generally testifies to secondary bacterial superinfection.
Pathognomonic Signs and Lesional Evolution
Lesional evolution generally follows a predictable pattern, although chronology can vary considerably from one individual to another. Initial phases are frequently accompanied by superficial crusts and exudate of variable composition, ranging from simple serous oozing to purulent discharge in case of superinfection.
A particularly evocative pathognomonic sign resides in the spontaneous extrusion of whitish chalky material through the epidermis. This phenomenon corresponds to transcutaneous elimination of calcium crystals and constitutes a diagnostic element of prime importance. Observation of this crystalline material, easily recognizable by its texture and characteristic coloration, often allows establishing the diagnosis with quasi-certainty.
Ulceration represents a common complication, particularly in severe or neglected forms. These ulcers present specific characteristics: elevated and indurated edges, anfractuous bed lined with granulation tissue, tendency to chronicization and resistance to conventional healing treatments.
During the resolution phase, observed mainly after correction of the underlying cause, lesions undergo a progressive transformation of their appearance. The inflammatory component gradually attenuates, translating into a decrease in perilesional erythema and local sensitivity. Coloration evolves toward duller grayish tonalities, testifying to the progressive normalization of local tissue processes. However, induration may persist for months, or even permanently in certain cases.
Infectious Complications and Evolution
Secondary bacterial superinfections constitute a quasi-constant complication, favored by alteration of the epidermal barrier and by the local inflammatory environment which compromises natural defense mechanisms. These superinfections can mask underlying lesions and significantly complicate diagnostic evaluation.
The bacterial flora involved classically comprises staphylococci and streptococci, but more resistant opportunistic germs can colonize chronic lesions. Precise identification of these pathogens by bacterial culture and antibiogram often proves necessary to adapt local or systemic antibiotherapy.
The presence of calcium material in tissues creates an environment particularly conducive to the development of bacterial biofilms, complex three-dimensional structures that protect microorganisms from the action of antimicrobials and host defense mechanisms. This particularity explains the frequent resistance to conventional antibiotic treatments and the tendency for superinfection recurrence.
Differential Diagnostic Strategy
Establishing the diagnosis of calcinosis relies mainly on recognition of characteristic clinical patterns and analysis of anamnestic context. Chronic or recurrent deep pyoderma constitutes the main diagnostic pitfall, especially since both conditions can coexist and mutually potentiate.
Several elements allow orienting the differential diagnosis: the particularly hard consistency of calcinosis lesions contrasts with the softer consistency of pyodermas, extrusion of chalky material constitutes a pathognomonic sign of calcinosis, and anamnestic context (corticotherapy, Cushing’s syndrome) strongly orients toward calcinosis.
Other conditions can occasionally lead to confusion: foreign body granulomas, calcified cutaneous tumors, infected sebaceous cysts, or chronic panniculitis. Taking into account all clinical and anamnestic elements generally allows resolving these diagnostic ambiguities.
Cutaneous cytology proves particularly valuable for characterizing concomitant bacterial superinfections and orienting local antimicrobial choices. Although not allowing formal confirmation of calcinosis diagnosis, this examination contributes to global evaluation of lesional state and guides complementary therapeutic decisions. Observation of calcium crystals during cytological examination can reinforce diagnostic suspicion.
In certain complex or atypical cases, cutaneous histopathology may prove necessary to confirm the diagnosis and precisely characterize the type of calcinosis. This examination allows objectifying the presence of calcium deposits in dermal structures and evaluating the intensity of associated inflammatory reaction.
Current Therapeutic Strategies and Limitations
Fundamental Principle: Correction of Primary Cause
Therapeutic management of cutaneous calcinoses relies on a cardinal principle: identification and correction of the underlying cause constitute the fundamental step, conditioning the entire prognosis. This etiological approach takes precedence over any symptomatic consideration and determines the chances of lesional regression in the medium and long term.
In the majority context of iatrogenic hypercortisolism, this approach implies progressive and carefully controlled cessation of corticotherapy. This decrease must be adapted to the duration of previous treatment, doses used and the initially treated pathology. Too abrupt withdrawal exposes to the risk of iatrogenic adrenal insufficiency, particularly formidable after prolonged treatments at high doses.
The decrease protocol must take into account the biological half-life of used corticosteroids and the duration of hypothalamic-pituitary-adrenal axis suppression. Generally, a reduction of 25 to 50% of the dose every two weeks constitutes an acceptable compromise between efficacy and safety. In certain cases, substitution with alternative anti-inflammatories (immunosuppressants, biotherapies) can facilitate corticosteroid withdrawal.
Regression of cutaneous lesions is indeed observed after correction of the primary cause, but its extent remains unpredictable, oscillating between complete resolution and only partial improvement. Factors influencing this variability include the duration of lesion evolution before treatment, the intensity of corticosteroid exposure, and individual tissue regeneration capacity.
Local Care and Complication Management
Local antisepsis constitutes an essential therapeutic pillar in the management of cutaneous calcinoses. Rigorous application of topical antiseptics, particularly chlorhexidine at adapted concentration (0.5 to 2% according to local tolerance), proves fundamental for controlling secondary bacterial superinfections and maintaining a local environment conducive to healing.
This preventive approach allows limiting infectious complications that can mask the natural evolution of lesions and compromise evaluation of therapeutic efficacy. Chlorhexidine presents the advantage of excellent local tolerance and broad antimicrobial spectrum, including gram-positive and gram-negative bacteria usually involved in cutaneous superinfections.
Other antiseptics can be used alternatively or in rotation: povidone iodine, particularly effective but potentially irritating, low-concentration chlorine derivatives, or silver-based antiseptics for resistant ulcerated lesions. Alternating antiseptics can prevent the development of bacterial resistance.
Systemic antibiotherapy remains rarely indicated in routine management, except in cases of proven septicemia, extensive superinfection with systemic signs, or failure of local antiseptic treatments. When necessary, antibiotic choice must be guided by bacterial culture and antibiogram, given the frequency of resistance in this context.
Use of keratomodulating and emollient agents can facilitate softening of hyperkeratotic and crusty areas, thus improving animal comfort and favoring elimination of superficial debris. These topicals, including urea, low-concentration salicylic acid, or ceramides, also contribute to progressive restoration of cutaneous suppleness in areas peripheral to lesions.
Occlusive dressings can be beneficial for maintaining local hydration and protecting ulcerated lesions from mechanical trauma. However, their use requires careful monitoring to prevent excessive maceration that would favor superinfections.
Selective Surgical Approaches
Surgical excision retains its relevance for single, very localized lesions that are sources of major discomfort for the animal. This therapeutic option must be carefully evaluated according to several criteria: size and location of lesions, general condition of the animal, technical feasibility of excision, and healing prospects.
Advantages of the surgical approach include immediate resolution of problematic lesions, possibility of confirmatory histopathological analysis, and rapid improvement of animal comfort. However, disadvantages include anesthetic risks, particularly in animals presenting hypercortisolism with its cardiovascular and metabolic complications, and risk of healing disorders related to hypercortisolism.
CO2 laser can constitute an interesting alternative for certain particular situations. This technique presents the advantage of better hemostasis, superior cutting precision, and potentially improved healing. However, its accessibility remains limited and its use requires specific technical expertise.
Reconstructive surgery techniques may prove necessary for extensive lesions, particularly in areas where primary closure proves impossible. Local cutaneous flaps or grafts can be considered, although hypercortisolism often compromises graft uptake.
Dimethyl Sulfoxide: Controversial Efficacy and Limits
DMSO, an organic solvent with recognized anti-inflammatory properties, notably through free radical scavenging and inhibition of certain inflammatory pathways, benefits from historical use in calcinosis treatment. Its remarkable tissue penetration capacity, linked to its particular molecular structure, theoretically makes it an interesting candidate for this topical indication.
DMSO’s physicochemical properties explain its potential interest: low molecular weight facilitating transcutaneous diffusion, capacity for solubilizing numerous substances, anti-inflammatory properties through inhibition of free radical polymerization, and local analgesic effect through blocking of small-caliber nerve fibers.
However, its real efficacy remains to be demonstrated by rigorous controlled studies. The difficulty of evaluation stems from the fact that calcinoses can regress spontaneously once the primary cause is corrected, making complex the attribution of observed improvements to DMSO treatment. The exact mechanism of action in this context remains enigmatic: direct dissolution of calcium crystals, non-specific anti-inflammatory effect, improvement of local microcirculation, or simple placebo effect.
The absence of standardized protocol constitutes a major limitation that compromises objective evaluation of this therapeutic. Literature reports extremely variable therapeutic regimens, from three to four daily applications for two weeks maximum to one bi-weekly application over several weeks according to certain case reports. Muller & Kirk recommendations advocate two daily applications without duration precision, while other sources suggest one application every two days over several weeks.
This protocol heterogeneity reflects the absence of solid evidence-based data and the empirical nature of this use. Establishing a standardized protocol would require controlled clinical studies randomized comparing different therapeutic regimens to placebo.
Potential side effects impose particular vigilance and limit use of this molecule. For the applicator, wearing thick rubber gloves proves essential to avoid percutaneous absorption that can cause undesirable systemic effects. Vapor inhalation must also be avoided, requiring adequate ventilation of application premises.
In the treated animal, various local reactions have been reported: immediate burning sensation after application, transient erythema and edema, cutaneous dryness and desquamation, and in certain cases, paradoxical aggravation of local inflammation. Ocular effects in case of prolonged use or high dose include crystalline lens modifications and accommodation disorders. Non-specific general malaise may also occur, particularly during applications on extensive surfaces.
Data concerning possible nephrotoxicity or hepatotoxicity remain contradictory and insufficiently substantiated. Certain reports suggest alterations of hepatic parameters during prolonged use, while other studies find no significant systemic toxicity. This uncertainty imposes clinical and biological monitoring in animals treated for prolonged periods.
In case of use on large cutaneous surfaces, a prudent approach consisting of treating small areas sequentially is strongly recommended to minimize risks of systemic absorption and adverse effects. This strategy also allows evaluating local tolerance before extending treatment.
Diversity of Human Pharmacological Approaches
Human medicine has developed a substantial therapeutic arsenal for calcinosis management, targeting different pathophysiological mechanisms. These multiple approaches offer interesting perspectives for transposition in veterinary medicine, although no systematic evaluation has yet been undertaken in the canine species. The absence of a universally effective standard treatment in human medicine underlines the complexity of this pathology and the necessity for individualized approaches.
Human therapeutic strategies aim at several mechanisms: inhibition of calcium crystal formation, improvement of their solubilization in tissues, reduction of local and systemic inflammation, or modulation of calcium and phosphate metabolic pathways. This multimodal approach allows adapting treatment to predominant pathophysiological mechanisms in each particular case.
Diltiazem, a calcium antagonist
It inhibits intracellular calcium entry through blockade of L-type voltage-dependent calcium channels. This action could theoretically limit tissue calcification processes by reducing availability of intracellular calcium for crystal formation. The human dosage of 2 to 4 mg/kg/day presents limited toxicity, mainly cardiovascular (hypotension, bradycardia), but safety in prolonged use in dogs remains undocumented.
Diltiazem use in veterinary cardiology, notably for feline hypertrophic cardiomyopathy treatment, suggests acceptable tolerance. However, dosage adaptation and side effect monitoring would require specific studies for calcinosis indication.
Bisphosphonates: Macrophage Activity Modulators
Bisphosphonates (alendronate, pamidronate, etidronate) exert their action through inhibition of macrophage activity and bone calcium resorption. These molecules, structural analogs of pyrophosphate, preferentially bind to hydroxyapatite and interfere with mineralization and demineralization processes.
Their efficacy has been documented in several human connective tissue diseases, particularly scleroderma and dermatomyositis, where they significantly reduce the size and number of calcium deposits. The mechanism of action probably involves inhibition of tissue macrophage activity that actively participates in dystrophic calcification processes.
Potential side effects include hypocalcemia, sometimes severe and requiring supplementation, post-administration fever (particularly with intravenous forms), and mandibular osteonecrosis, a rare but serious complication observed mainly with third-generation bisphosphonates.
In canine veterinary medicine, their use currently remains limited but certain molecules like pamidronate are used for managing metastatic bone pain or tumor osteolysis. This limited clinical experience suggests acceptable tolerance, but would require adaptation for cutaneous calcinosis indication.
Chelating Agents and Solubility Modulators
Sodium thiosulfate presents the property of increasing calcium solubility through formation of soluble complexes with calcium ions. This molecule can be used topically, intralesionally or intravenously according to indications and tolerance. Its mechanism of action involves direct chelation of tissue calcium and improvement of its mobilization toward systemic circulation.
Its notable systemic toxicity comprises nausea and vomiting, potentially severe hypocalcemia, and various electrolyte disorders including hypomagnesemia and hypokalemia. These adverse effects impose close biological monitoring during systemic use.
Although no current use is reported in dogs, research could be considered for local use as gel or topical solution. This approach would minimize risks of systemic effects while preserving the desired local effect.
Aluminum hydroxide acts as an intestinal phosphate binder and reduces digestive absorption of the latter. This indirect action on phosphocalcium metabolism could present interest in metastatic forms of calcinosis. Human doses of 1.8 to 2.4 g/day suggest potential use in animals, but this application remains unvalidated in the calcinosis context.
Use of this molecule in veterinary medicine for hyperphosphatemia management in chronic renal insufficiency suggests acceptable tolerance. However, dosage adaptation and specific efficacy evaluation would require dedicated studies.
Antibiotics with Anti-calcifying Properties
Minocycline presents particularly interesting multiple properties: inhibition of matrix metalloproteinases (MMPs) that participate in tissue remodeling processes, direct calcium chelation through its functional groups, and inflammation reduction through inhibition of microglial activation and pro-inflammatory cytokine production.
Human dosage of 50 to 100 mg/day opens perspectives for use in dogs, especially since this molecule already exists in certain veterinary dermatological indications, notably for treating pyodermas with resistant germs. This pre-existing clinical experience would facilitate dosage adaptation and side effect monitoring.
Matrix metalloproteinases play a crucial role in dystrophic calcification processes by degrading extracellular matrix and releasing factors favoring mineralization. Their inhibition by minocycline could therefore present direct therapeutic interest.
Ceftriaxone offers a potentially beneficial side effect through matrix metalloproteinase inhibition, calcium chelation by its β-lactam groups, and non-specific anti-inflammatory action. However, responsible antibiotic prescription rules, particularly strict for third-generation cephalosporins, formally prohibit considering its use in veterinary medicine for this non-infectious indication.
This regulatory restriction underlines the importance of developing non-antibiotic alternatives to avoid contributing to antibiotic resistance while exploring innovative therapeutic pathways.
Phosphocalcium Metabolism Modulators
Probenecid increases renal phosphate excretion through inhibition of its tubular reabsorption. Its beneficial effect has been documented in juvenile dermatomyositis, where it significantly reduces cutaneous calcium deposits. This molecule is not currently used in veterinary medicine, but its mechanism of action could present interest for metastatic forms of calcinosis.
Adaptation of this approach in veterinary medicine would require preliminary pharmacokinetic studies to determine appropriate dosages and evaluate tolerance in the canine species. Human side effects mainly include digestive disorders and hypersensitivity reactions.
Colchicine
It presents specific anti-inflammatory properties through inhibition of microtubule polymerization and modulation of neutrophil activation. Its use in veterinary medicine remains little developed, but some applications exist, notably for Shar-Pei familial fever treatment.
A particularly encouraging case report documented clear improvement of calcinosis lesions in a dog treated with colchicine at the dose used for Shar-Pei familial fever (approximately 0.01 to 0.03 mg/kg/day). Although this data remains very preliminary and constitutes only an isolated observation, it illustrates the potential interest of targeted anti-inflammatory agents and underlines the necessity of conducting clinical studies to rigorously evaluate these new options.
Colchicine’s mechanism of action in this context could involve inhibition of inflammasome activation, multiprotein complexes responsible for IL-1β and IL-18 maturation, key cytokines in dystrophic calcification processes.
This preliminary observation with colchicine opens particularly interesting perspectives as it demonstrates the feasibility of a specific pharmacological approach in veterinary medicine. The apparently satisfactory tolerance and observed clinical improvement justify implementing more rigorous clinical studies.
Colchicine’s advantage resides in its well-established safety profile in dogs, thanks to its use in Shar-Pei familial fever, and in its specific mechanism of action on inflammatory pathways involved in dystrophic calcification.
Practical Recommendations and Decisional Algorithm
Structured Diagnostic Approach
Early recognition of calcinoses relies on a diagnostic triptych: identification of characteristic clinical patterns, analysis of anamnestic context and exclusion of main differential diagnoses. This systematic approach allows avoiding diagnostic delays that compromise therapeutic efficacy and optimizing early management.
Anamnestic questioning must be particularly meticulous, systematically seeking antecedents of corticotherapy (molecule, dosage, duration, administration route), signs of spontaneous hypercortisolism (polyuria-polydipsia, polyphagia, bilateral symmetrical alopecia, amyotrophy, abdominal distension), and pathologies likely to cause phosphocalcium metabolism disorders (chronic renal failure, malignant tumors, hyperparathyroidism).
Clinical examination must be systematic and complete, not limiting itself to cutaneous lesions but also seeking associated systemic signs. Careful palpation of the entire integument allows identifying nascent or asymptomatic lesions that orient toward more diffuse involvement.
Evaluation of the underlying cause must be exhaustive and oriented by epidemiological data. Investigation of hypercortisolism, whether iatrogenic or spontaneous, constitutes absolute priority given its dominant frequency. This evaluation includes basal cortisol measurement, dexamethasone suppression tests, and possibly endogenous ACTH dosage to differentiate pituitary and adrenal forms.
Exploration of chronic renal failure is imposed secondarily, particularly in elderly animals or those with nephropathy antecedents. This evaluation comprises serum creatinine and urea measurement, complete urine analysis, and evaluation of phosphocalcium metabolism (calcemia, phosphatemia, parathormone if available).
Hierarchical Therapeutic Strategy
Correction of the primary cause remains the fundamental inescapable step, conditioning the entire medium and long-term prognosis. This etiological approach must be privileged over any symptomatic consideration, as it directly determines chances of spontaneous lesional regression.
In the majority case of iatrogenic hypercortisolism, progressive cessation of corticotherapy must be planned individually according to previous treatment duration, doses used, and initially treated pathology. A progressive decrease protocol over 4 to 8 weeks minimizes risks of iatrogenic adrenal insufficiency while allowing recovery of the hypothalamic-pituitary-adrenal axis.
For spontaneous hypercortisolism, specific treatment (trilostane, mitotane) or surgical (adrenalectomy, hypophysectomy) must be undertaken according to established recommendations for these pathologies. Calcinosis management then becomes secondary and generally follows hypercortisolism improvement.
Local antiseptic care constitutes an indispensable complement to prevent infectious complications and maintain integrity of surrounding tissues. This preventive approach allows preserving optimal conditions for spontaneous healing and avoiding aggravating factors.
The recommended antiseptic protocol comprises twice-daily cleaning of lesions with 0.5-2% chlorhexidine solution according to local tolerance, followed by careful drying and possible application of an emollient agent. This routine must be maintained until complete lesion resolution.
Use of specific treatments like DMSO must be reserved for severe or resistant cases, scrupulously respecting employment precautions and informing owners of uncertainties concerning real efficacy. A sequential approach, treating small areas successively, minimizes risks of systemic effects while allowing evaluation of local tolerance.
Monitoring Criteria and Prognostic Indicators
Evaluation of lesional evolution requires standardized objective criteria to allow reproducible assessment of therapeutic efficacy. Serial medical photography constitutes a valuable tool for documenting morphological modifications and quantitatively assessing evolution of lesional surface.
Parameters to monitor include lesion size (bidimensional measurement), their appearance (coloration, relief, induration), presence of complications (ulceration, calcium material extrusion, superinfection), and animal’s general condition (pruritus, pain, functional discomfort).
A composite lesional score integrating these different parameters would facilitate standardized evaluation and inter-individual comparison. This score could include a 0 to 3 rating for each parameter (size, induration, inflammation, ulceration) with a maximal global score orienting toward severity.
Monitoring of potential complications, particularly infectious ones, imposes regular clinical examinations at intervals adapted to initial severity. A weekly rhythm for severe forms, bi-weekly for moderate forms, and monthly for minor forms constitutes a reasonable compromise between optimal monitoring and practical constraints.
Repeated cutaneous cytology allows early detection of bacterial superinfections and adjustment of antiseptic protocols. This microbiological monitoring proves particularly important for ulcerated or exudative lesions that present increased infectious risk.
Therapeutic Failure Management
Absence of improvement after 6 to 8 weeks of well-conducted etiological treatment must make one reconsider the diagnosis or seek unknown aggravating factors. This situation imposes complete reevaluation including verification of effective correction of the primary cause and investigation of comorbidities.
Causes of therapeutic failure include persistence of residual hypercortisolism (insufficient decrease, unrecognized spontaneous hypercortisolism), presence of uncontrolled infectious complications, or existence of intrinsic resistance factors (excessive chronicity, healing disorders).
In these therapeutic impasse situations, use of adjuvant treatments inspired by human medicine can be considered in the absence of alternatives. This compassionate approach requires informed consent from owners about the experimental nature of these treatments and the necessity for reinforced monitoring.
Prevention and Education
Primary prevention of iatrogenic calcinoses relies on reasonable use of corticosteroids: prescription at minimal effective dose, limitation of treatment duration, search for therapeutic alternatives whenever possible, and regular clinical monitoring of patients under prolonged corticotherapy.
Owner education plays a crucial role in early lesion detection and therapeutic compliance. Advice includes daily monitoring of cutaneous condition, recognition of alarm signs requiring urgent consultation, and importance of compliance with prescribed therapeutic protocol.
Continuing education of practitioners on latest diagnostic and therapeutic advances would allow improving management quality and reducing diagnostic delays. This training should include recognition of atypical forms and knowledge of new therapeutic options.
Conclusion and Future Perspectives
Canine cutaneous calcinosis represents an exemplary model of dermatological pathology requiring a multidisciplinary approach integrating dermatology, endocrinology and internal medicine. Deep understanding of underlying pathophysiological mechanisms and precise identification of etiological causes constitute the foundations of optimal management that directly conditions functional and aesthetic prognosis.
The overwhelming predominance of hypercortisolism in canine calcinosis etiology, contrasting with the diversity of causes observed in human medicine, underlines the epidemiological particularities of this condition in the canine species. This etiological specificity relatively simplifies the diagnostic approach but must not neglect minority forms that require adapted therapeutic strategies.
Current limitations of the veterinary therapeutic arsenal contrast strikingly with the richness of options developed in human medicine. This disparity underlines the urgency of developing translational research programs to evaluate efficacy and safety of promising pharmacological approaches in the canine species. Human experience offers a considerable reservoir of therapeutic innovations that would merit adaptation and validation in veterinary medicine.
The absence of controlled clinical studies remains the main limitation to therapeutic progress in this field. This methodological gap perpetuates the use of empirical treatments and limits the ability to establish evidence-based recommendations. Implementation of multicenter randomized clinical trials constitutes an imperative to advance knowledge and improve quality of care.
Fundamental research on molecular mechanisms of canine cutaneous calcification could reveal species-specific particularities and identify new therapeutic targets. This mechanistic approach is indispensable for developing rational and effective treatments.