The latest annual GEDAC (Group for the Study of Dermatology in Companion Animals) conference held in Ajaccio provided an opportunity for our colleague Vincent Bruet to review perianal fistulas, their pathogenesis, clinical manifestations, and current therapeutic approaches.
Perianal fistulas in dogs represent a potentially debilitating dermatological condition that, without appropriate and prompt treatment, can lead to irreversible changes in the rectum and anus, sometimes resulting in euthanasia. This pathology, also known as anal furunculosis, primarily affects German Shepherd dogs but can affect other breeds and manifests as fistulous tracts or ulcerations around the anus.
Long considered to be linked to anatomical factors, the pathogenesis of perianal fistulas is now recognized as primarily immune-mediated. Therapeutic management has also evolved from an essentially surgical approach to a primarily medical strategy. This article provides a synthesis of current knowledge on this condition and presents therapeutic recommendations based on a systematic analysis of the scientific literature.
Pathogenesis and Clinical Aspects of Canine Perianal Fistulas
A Complex and Multifactorial Etiology
The exact pathogenesis of perianal fistulas remains partially elucidated, despite numerous studies. Historically, several hypotheses have been put forward to explain the occurrence of this condition. The high incidence in German Shepherds initially suggested a genetic link, but no specific genetic marker has been identified to date. Similarly, a low tail carriage was considered a predisposing factor, a theory now refuted since other breeds are affected and tail amputation is not effective as a treatment.
Recent research points to an immune-mediated pathogenesis. Changes in the local inflammatory response have been demonstrated, including infiltration by CD31+ T lymphocytes and increased expression of cytokines associated with type 1 helper T lymphocytes in the lesional skin. These mechanisms likely involve:
- Local inflammation mediated by T lymphocytes
- Abnormal macrophage activation, with overexpression of matrix metalloproteinases MMP-9 and MMP-13
- A dysfunctional immune response to microbes in the perianal region
- Possible healing abnormalities in the perianal area
- A potential food hypersensitivity reaction, although this hypothesis remains to be confirmed
A genetic study has shown an association between the MHC class II allele DLA-DRB1*00101 and an increased risk of developing perianal fistulas in German Shepherds. Class II molecules of the major histocompatibility complex participate in antigen presentation and T lymphocyte activation, which reinforces the hypothesis of an immune-mediated pathogenesis. Another study revealed a potential association with the ADAMTS16 and CTNND2 gene regions, the latter also being associated with Crohn’s disease and ulcerative colitis in humans.
Clinical Presentation and Signalment
Breed Predisposition and Age of Onset
Perianal fistulas primarily affect German Shepherd dogs, which account for more than 80% of affected dogs according to some studies. However, several other breeds can be affected, including:
- Beagles
- Border Collies
- Australian Shepherds
- Irish Setters
- Chesapeake Bay Retrievers
- Leonbergers
- Staffordshire Bull Terriers
- Shih Tzu
- Welsh Corgis Pembroke
- Boxers
- Labrador Retrievers
- Mixed-breed dogs
The age of onset is generally between young adulthood and middle age, with a range of 2 to 9 years for most patients. No predisposition related to sex or sterilization status has been clearly established, although one study suggested an increased risk in intact male German Shepherds compared to castrated males.
Lesional Aspects and Clinical Manifestations
Characteristic lesions of perianal fistulas include:
- Sinuses or fistulous tracts around the anus
- Perianal ulcerations
- Erosions
- Pustules in less severe cases
- Rectal fistulas more rarely
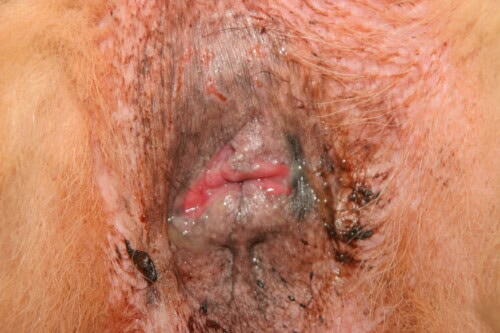
Moderate perianal fistula lesions
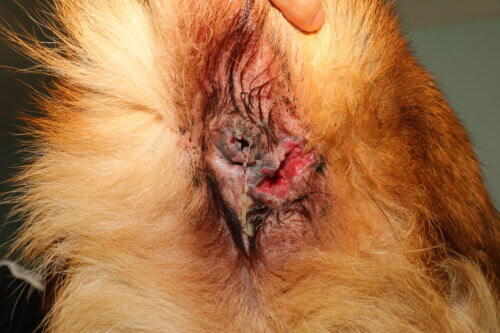
Severe lesions primarily affecting the lower right quadrant
It is important to note that, contrary to what the term “perianal fistula” suggests, the cutaneous sinus tracts generally do not communicate with the rectal lumen, unlike fistulas observed in Crohn’s disease in humans. These lesions can be single or multiple, and their severity ranges from mild to severe, with often significant associated pain.
Clinical signs reported by owners include:
- Tenesmus (straining to defecate)
- Hematochezia (presence of blood in stools)
- Dyschezia (pain during defecation)
- Passage of small, frequent stools
- Excessive perianal licking
- Purulent discharge
- Constipation
- Coprophagia
- Anorexia
- Diarrhea
- Fecal incontinence
- Weight loss
- Lethargy
These clinical manifestations can be highly debilitating and have a significant impact on the animal’s quality of life. An association between perianal fistulas and colitis has been demonstrated, and some affected dogs also present with soft or mucoid stools, diarrhea, and increased defecation frequency.
Diagnostic Approach
Clinical Examination and Differential Diagnosis
The diagnosis of perianal fistulas is primarily based on clinical presentation and physical examination, particularly in German Shepherds. A thorough examination of the perianal region and a digital rectal examination are essential to assess the extent of lesions and identify any associated abnormalities. Due to the pain and discomfort caused by this condition, sedation may be necessary to perform a complete rectal examination.
Probing the fistulous tracts with a cotton-tipped applicator or similar instrument helps determine their depth. Clinical monitoring can be performed by measuring the depths of these tracts. Depending on the severity and duration of the disease, rectal examination may reveal no internal abnormalities or severe fibrosis, identifiable by thickening of the perianal region with possible anal strictures.
For all patients, thorough palpation and expression of the anal sacs should be performed to rule out an anal gland abscess and to determine if the perianal fistulas communicate with the anal sacs. Additionally, fistulous tracts can be flushed with a catheter and sterile saline to determine if they communicate with each other or if there is anal sac involvement.
The differential diagnosis varies considerably depending on the clinical signs present:
- In cases of perianal pruritus only: allergic dermatitis (atopic dermatitis and adverse food reaction), anal sac abscesses
- In cases of tenesmus and hematochezia without visible fistulous tracts: gastrointestinal conditions (inflammatory bowel disease, intestinal parasites, rectal strictures)
- Ulcerated perianal neoplasia (perianal adenoma or adenocarcinoma)
- Cutaneous mucocutaneous lupus erythematosus (CMLE)
It is important to note that German Shepherds are also predisposed to CMLE, which can present with anal or perianal lesions. Perianal fistulas generally differ from CMLE by distinct sinuous tracts or crater-like ulcers, while CMLE is associated with more confluent erosions, ulcers, erythema, and crusts.
If clinical differentiation between perianal fistulas and CMLE is difficult, skin biopsies for histopathology may be useful. Similarly, if the dog belongs to a less commonly affected breed, other causes based on clinical signs should be ruled out first.
Histopathological Evaluation
Although histopathology is rarely performed for the diagnosis of canine perianal fistulas, it may be considered in cases of unusual clinical presentation or in a less commonly affected breed. Histopathological features include:
- Periadnexal inflammation with or without furunculosis
- Pronounced hidradenitis
- Periadnexal fibrosis
- Ulceration
- Formation of epithelial-lined sinus tracts in the dermis
Inflammatory infiltrates, composed of neutrophils, lymphocytes, plasma cells, and macrophages, can be observed in the sinus tracts. Deeper lesions may also be associated with pyogranulomatous cellulitis and lymphoid follicles.
Colonic biopsies from dogs with perianal fistulas may show histopathological signs of colitis; one study found histopathological changes consistent with a diagnosis of colitis in 9 out of 18 dogs with perianal fistulas.
Therapeutic Approaches to Perianal Fistulas
Surgical Management: A Limited Role
Evolution of Surgical Practices
The management of perianal fistulas has evolved considerably over time. From the 1940s to the 1970s, these lesions were considered an exclusively surgical pathology. Procedures typically involved resection of all affected tissues, with or without anal sac removal. This procedure could involve focal resection or extend to complete circumferential resection (360 degrees) with anoplasty.
Various surgical techniques have been described:
- Block excision followed by surgical reconstruction
- Laser excision (especially ND:YAG laser)
- Cryotherapy to destroy diseased tissue
- Tail base amputation (when fistulas were considered associated with low tail carriage)
Typically, patients received a fecal softener after surgery to reduce pressure during bowel movements and allow the area to heal.
Limitations and Complications of the Surgical Approach
The use of surgery as the exclusive therapy presents several major problems:
- High recurrence rate (reported in over 50% of dogs in one study)
- Absence of complete resolution in many cases
- Significant surgical complications:
- Wound dehiscence
- Flatulence
- Fecal incontinence
- Diarrhea
- Tenesmus
- Constipation
- Rectal strictures
Study results vary, but surgery has been reported to be ineffective in 6% to 21% of dogs, with some being abandoned or euthanized as a result.
Complementary Surgical Approach
More recently, several studies have described the use of surgery after the efficacy of immunosuppressive therapies has plateaued. This combined approach, where medical treatment precedes surgical intervention, appears to yield better results than surgery alone.
In this strategy, surgical treatment intervenes:
- When medical improvement has plateaued
- To manage persistent lesions after improvement achieved with medical treatment
- In animals still experiencing discomfort or pain (impaired quality of life)
- For budgetary reasons, when long-term medical treatment is too expensive
Surgical procedures performed in this context include:
- Excision of persistent fistulas
- Saccultectomy
- Cryptectomy
- Suture of any rectal fistulas
Immunomodulatory Therapies: Cornerstone of Treatment
Calcineurin Inhibitors
Cyclosporine A
Cyclosporine A is considered one of the reference treatments for canine perianal fistulas. Its mechanism of action involves binding to the intracellular protein cyclophilin-1, inhibiting calcineurin. This inhibition prevents the dephosphorylation of activated T lymphocyte nuclear factor and the subsequent production of pro-inflammatory cytokines, especially IL-2. Reduced IL-2 production leads to decreased growth and activation of T lymphocytes.
Several studies, including randomized and controlled clinical trials, have demonstrated the effectiveness of cyclosporine A. In a randomized clinical trial comparing cyclosporine A to placebo in German Shepherds with perianal fistulas, complete resolution of lesions was reported in 17 out of 20 dogs (85%) receiving cyclosporine A after 16 weeks of treatment. The mean total surface area and depth of lesions decreased by 78% and 62% respectively after 4 weeks of therapy.
The doses of cyclosporine A studied for the treatment of perianal fistulas vary considerably, ranging from 1.5 mg/kg every 24 hours to 10 mg/kg every 12 hours. In general, higher doses are associated with better results, with efficacy being dose-dependent. Modified or microemulsified formulations of cyclosporine A have superior bioavailability in dogs and should be preferentially used. The bioavailability of cyclosporine A is also reduced by the presence of food; therefore, it is best administered on an empty stomach (2 hours before or after a meal).
The cost of cyclosporine A, especially at high doses or for large dogs, can be prohibitive. The co-administration of cyclosporine A and ketoconazole can inhibit cyclosporine A metabolism by hepatic cytochrome P450 microenzymes and improve its bioavailability via inhibition of intestinal P-glycoprotein (thus decreasing cyclosporine A transport to the intestinal lumen). The co-administration of the two drugs can improve cyclosporine A bioavailability by 75% or more, depending on the ketoconazole dose, and allow for a reduction in the cyclosporine A dose.
The combination of cyclosporine A (at doses of 1 mg/kg/day to 5.5 mg/kg/day) and ketoconazole (at doses of 5.1 mg/kg/day to 11 mg/kg/day) has proven effective in clinical trials with complete resolution of lesions in 93% of dogs in 16 weeks, 100% of dogs in 3 to 10 weeks, and 67% of dogs with an average resolution time of 13.9 weeks.
Recurrences can occur when immunomodulatory treatment is discontinued. For this reason, after complete resolution of lesions (which usually requires 8 to 12 weeks of treatment), it is recommended to gradually reduce the doses of cyclosporine A and ketoconazole to the lowest effective dosage and administration frequency.
Tacrolimus
Tacrolimus 0.1% ointment has also been shown to be effective for the treatment of canine perianal fistulas, although randomized and controlled clinical trials have not been performed. In one study of 10 dogs treated with 0.1% tacrolimus ointment applied to the perianal skin twice daily for 16 weeks, 5 dogs achieved complete resolution of lesions.
Tacrolimus is more suitable for topical application than cyclosporine A due to its lower molecular weight, which allows for better absorption through the epidermis. Due to the potential discomfort associated with application, tacrolimus ointment is generally recommended for dogs with mild lesions or for those with more severe lesions after complete or partial resolution with oral cyclosporine A (most often in combination with ketoconazole).
Some dogs may transition to topical tacrolimus ointment alone for maintenance and prevention of perianal fistula recurrence. The main advantage of this topical treatment is to avoid systemic immunosuppression.
A larger study showed complete remission over 16 weeks in 15 out of 19 patients (79%) and marked improvement in the remaining 4 patients when tacrolimus was used in combination with prednisolone, a novel protein diet, and a short course of metronidazole. When tacrolimus is administered alone, complete remission has only been reported in 50% of patients.
Glucocorticoids
Immunosuppressive doses of prednisolone (2 to 4 mg/kg PO q24h) have been described as effective, with 33.3% of patients achieving complete remission, 33.3% showing clinical sign improvement, and 33.3% showing no improvement. An advantage of prednisolone therapy is its rapid action compared to other immunosuppressive therapies; the problem with prednisolone therapy lies in long-term side effects.
Due to the need for continuous administration of immunomodulatory agents for management, as well as the risk of side effects with long-term corticosteroid administration, corticosteroids are generally not used alone for the management of canine perianal fistulas. However, they may be useful as part of a multimodal management, especially in the initial phase for their rapid anti-inflammatory action.
Other Immunomodulators
Azathioprine
Azathioprine, in combination with metronidazole, has been shown to be effective in reducing the size of perianal fistulas prior to surgical removal of residual disease. In reported cases, azathioprine was continued for 2 to 6 weeks after surgery. Another study showed that monotherapy with azathioprine led to complete remission in slightly more than half of the dogs receiving it. However, azathioprine can cause significant side effects (e.g., severe myelosuppression, hepatotoxicity, pancreatitis), which must be considered when using this drug.
In a study by Harkin et al., 14 dogs with perianal fistulas were treated with azathioprine alone. After 16 weeks of treatment, 8 out of 14 dogs (57%) achieved complete resolution of lesions.
Mycophenolate Mofetil
To the author’s knowledge, there is only one report on the use of mycophenolate mofetil for the management of perianal fistulas in veterinary literature; this dog was treated for 4 weeks with no improvement in lesions.
Alternative and Emerging Therapies
Mesenchymal Stem Cell Injections
Mesenchymal stem cell injections, administered directly into the fistulas, have proven effective in humans with fistulizing Crohn’s disease. Mesenchymal stem cells have immunomodulatory activity, decreasing T lymphocyte and dendritic cell proliferation and activation and increasing the production of regulatory T cells.
The efficacy of mesenchymal stem cell injections for canine perianal fistulas has been reported for a small number of dogs. Six dogs with perianal fistulas that had not responded to standard-dose cyclosporine A therapy for at least 6 months of administration were included in an open trial and received a single injection of human embryonic stem cell-derived mesenchymal stem cells into the perianal lesions. Dogs were followed for 6 months after injection; all dogs showed resolution of sinus tracts or ulcers 3 months after stem cell injection. Two dogs had recurrence of perianal fistulas 6 months after injection.
After 1 to 2 months, the average cyclosporine dose could be reduced to 3.59 mg/kg/day (at 3 months) compared to 8.22 mg/kg/day initially. Although mesenchymal stem cell injections are a promising treatment option for canine perianal fistulas, additional controlled studies are needed to apply these results to larger populations of affected dogs and to determine the optimal frequency of injections.
Other Innovative Therapies
Several alternative therapies targeting wound healing have become popular as treatment options for perianal fistulas.
-
Platelet-rich plasma: Monotherapy with platelet-rich plasma administered as intralesional injections in one dog resulted in complete remission without recurrence after one year.
-
Fluorescent light energy: This therapy requires weekly treatment to help decrease inflammation and increase wound healing. A study of 4 cases showed a 74.3% decrease in lesional area after 6 weeks, although no complete healing was observed. The recent commercialization of fluorescent light energy has made it more readily available to veterinarians.
-
Oclacitinib: A recent case series reported the use of oclacitinib at doses higher than those used for atopic dermatitis (1.125 mg/kg BID and 0.88 mg/kg BID) with complete healing in both cases after 1 month of treatment. This drug acts by blocking the action of interferons and the JAK-STAT pathway, which could make it a relevant etiological treatment given the activation of interferons in perianal fistula lesions.
Adjuvant Treatments and Complementary Considerations
Management of Secondary Infections
Secondary infections may accompany perianal fistulas and should be treated appropriately, either with systemic medications or topical applications (chlorhexidine). Routine hygienic care of the perianal region can be beneficial (e.g., antiseptic baths 2 to 3 times a week, or even daily, depending on the severity of the lesions).
It is important to note that antibiotics alone do not appear to be effective for the management of perianal fistulas in dogs. Many dogs are treated with antimicrobials at the time of onset of clinical signs of perianal fistulas but continue to show disease progression.
A few studies have reported the use of metronidazole in combination with other therapies (azathioprine, tacrolimus ointment, prednisolone, and novel protein diet) for the treatment of dogs with perianal fistulas. In addition to its activity against anaerobic bacteria and protozoa, metronidazole may also have anti-inflammatory activity, particularly by promoting the differentiation of regulatory T cells. This anti-inflammatory activity may aid in the treatment of immune-mediated diseases, but the efficacy of metronidazole alone for the treatment of perianal fistulas is currently unknown.
Pain and Comfort Management
Dogs with active perianal fistulas may experience pain, especially during defecation. Fecal softeners should be considered, and in some cases, enemas may be necessary to alleviate tenesmus. Analgesia should be a priority for dogs with active lesions, but the potential for constipation with the use of certain analgesics, such as opioid agonists, should be considered when developing a pain management plan.
Dietary Considerations
A link between perianal fistulas and adverse food reactions has been identified. In one study, 18.8% of dogs with perianal fistulas also suffered from food allergies. It is unknown whether there is a direct correlation between these diseases or if the dogs simply had two concomitant diseases. Regardless, the use of a novel protein diet or a hydrolyzed diet has been beneficial for some patients.
Three studies have reported a positive clinical response to a novel protein diet in dogs with perianal fistulas. In one study, a fish and potato diet was administered long-term after surgical excision of sinus tracts and bilateral anal saccultectomy. In the second study, dogs received an exclusive venison and potato or fish and potato diet, but also initially received metronidazole, as well as 0.1% topical tacrolimus ointment and a tapering course of oral prednisolone for 16 weeks. In the third study, dogs received a commercial lamb and rice or vegetarian diet, but also simultaneously received a tapering course of oral prednisolone at an initial immunosuppressive dose.
Although placing an elimination diet using a novel protein or hydrolyzed protein food is recommended as part of the diagnostic workup for dogs with perianal fistulas, it is not clear from the available literature how many dogs can achieve long-term remission with dietary control alone.
Consensus Recommendations for the Management of Perianal Fistulas
Consensus Development Methodology
In the absence of consensus on the management of perianal fistulas, the scientific council of GEDAC (Group for the Study of Dermatology in Companion Animals) undertook to establish a review of the literature in order to arrive at recommendations for medical management.
The methodology adopted is based on the Strength of Recommendation Taxonomy (SoRT) developed by editors of general medical and emergency care journals in the United States. The Level of Evidence (LoE) was classified according to a three-level scale:
- LoE 1: Good quality evidence (meta-analysis, high-quality randomized controlled prospective study)
- LoE 2: Limited quality evidence (low-quality randomized controlled prospective study, controlled trial, case study versus control group, high-quality prospective study)
- LoE 3: Other evidence, including consensus guidelines, opinions, case studies
A systematic search of the literature published between 1980 and October 2023 was performed in the PubMed database. Articles in English and French were searched using specific keywords, and the following study types were initially selected: randomized controlled trials, clinical trials, cohort studies, and case series.
For the development of recommendations, a three-level scale was used:
- Level A: Strong recommendation, based on good quality, consistent evidence
- Level B: Moderate recommendation, based on inconsistent or limited quality evidence
- Level C: Weak recommendation, primarily based on expert opinion
Main Therapeutic Recommendations
Cyclosporine A
- The efficacy of cyclosporine is real (recommendation level A)
- Efficacy is dose-dependent and independent of clinical severity
- The minimum recommended dose is 5 mg/kg/day, which can go up to 10 mg/kg twice daily
- The effective dose should be maintained until clinical healing is achieved (level C)
- A duration of three months may be necessary to observe healing under cyclosporine
Cyclosporine-Ketoconazole Combination
- This combination is effective
- However, experts do not recommend this association to limit the risks of antifungal resistance and liver damage
Tacrolimus
In France, changes in legislation make the use of tacrolimus difficult to consider.
Prednisolone
- Experts do not recommend the sole use of prednisolone
- Corticotherapy is advised as part of a multimodal approach
- It is advisable not to exceed 1 mg/kg/day as an initial dose to limit adverse effects
- Topical corticotherapy could be interesting despite the lack of solid scientific data on this subject
Other Immunomodulators
Experts do not recommend the use of azathioprine, mycophenolate mofetil, oclacitinib, photobiomodulation, or stem cells due to a lack of reliable and repeatable data available to date.
Antibiotic Therapy
- Antibiotic therapy is not necessary to achieve healing
- Metronidazole, rarely used in studies, does not allow for evaluation of its impact on disease progression
- Experts do not recommend the systematic use of antibiotics
- Antibiotic therapy is recommended only in cases of rectal fistula or anal sac infection, based on bacterial culture results
Surgical Approach
- Experts recommend surgery as a second-line option
- This approach is indicated in an animal still experiencing discomfort or pain (impaired quality of life) after improvement with medical treatment
- The use of surgery may be conditioned by budgetary reasons or a stagnation of clinical improvement in an animal whose quality of life remains insufficient
Diet
- No scientific evidence supports the recommendation of novel protein or hydrolyzed protein foods
- By analogy with Crohn’s disease in humans and based on clinical experience, experts advise the implementation of a hypoallergenic diet (level C)
Monitoring and Therapeutic Adaptation
- It is recommended to evaluate medical treatment after 6 to 10 weeks and modify it according to the observed response
- After achieving healing or in the presence of moderate persistent lesions associated with good quality of life, treatment can be simplified or discontinued
- In the absence of scientific data, experts advocate for gradual and adapted simplification or cessation of treatment
Discussion and Perspectives
Parallels with Human Pathologies
Two human conditions, hidradenitis suppurativa and fistulizing Crohn’s disease, have been proposed as correlates of canine perianal fistulas. Like perianal fistulas in dogs, these two conditions are painful, debilitating, and can negatively impact patients’ quality of life.
Hidradenitis suppurativa is associated with nodules, abscesses, sinus tracts, and scars, primarily in intertriginous regions (armpit, groin, perianal region, and mammary region). It was initially proposed as a potential correlate of canine perianal fistulas due to similar histopathological features, including furunculosis, hidradenitis, and the formation of epithelial-lined sinus tracts. The pathogenesis of hidradenitis suppurativa has not been fully elucidated, but it is likely an immune-mediated condition with genetic susceptibility. Alterations in the cutaneous or intestinal microbiota may play a role in pathogenesis.
Perianal manifestations of Crohn’s disease are common, estimated to affect 25% to 33% of patients with Crohn’s disease. Perianal fistulas are more frequent in patients with colonic or rectal disease and can be simple (a single fistulous tract with one external opening) or complex (multiple fistulous tracts, perianal abscesses, or anal/rectal strictures). Unlike the typical disease in dogs, these are true fistulas that communicate with the intestinal lumen. The pathogenesis of perianal fistulas in Crohn’s disease is not fully understood but is thought to involve epithelial-mesenchymal transition and upregulation of matrix metalloproteinases and pro-inflammatory cytokines.
Similarities in the pathogenesis of hidradenitis suppurativa and Crohn’s disease have been demonstrated, particularly the infiltration of diseased tissues by type 17 helper T lymphocytes. Future studies should focus on better characterizing the underlying etiology of these three conditions and could help guide the development of future therapies, such as antibodies targeting specific cytokines, for perianal fistulas in dogs.
Future Perspectives and Research Avenues
Several research areas deserve particular attention to improve the management of canine perianal fistulas:
-
In-depth study of immune pathogenesis: A better understanding of the immune-mediated mechanisms involved could lead to the development of targeted therapies, such as the use of JAK inhibitors (oclacitinib), whose efficacy has been suggested by some preliminary studies.
-
Characterization of the cutaneous and intestinal microbiome: Exploring the role played by the microbiome in the development and persistence of perianal fistulas could open new therapeutic avenues, such as the use of probiotics or fecal transplantation.
-
Optimization of therapeutic protocols: Controlled studies are needed to determine optimal doses, treatment durations, and weaning strategies for various therapeutic options.
-
More standardized quality of life assessment: The development of specific tools to assess the impact of perianal fistulas and their treatments on dogs’ quality of life would allow for a more animal-welfare-focused approach.
-
Development of innovative therapies: Encouraging preliminary results obtained with mesenchymal stem cells, platelet-rich plasma, or fluorescent light energy deserve further investigation through controlled studies on larger populations.
-
Study of links with food allergies: Clarifying the potential links between perianal fistulas and adverse food reactions would allow for the optimization of nutritional approaches.
- Identification of predictive biomarkers: Research into biological markers that can predict treatment response or recurrence risk would improve therapeutic personalization.
Importance of the Individualized Approach
The management of canine perianal fistulas requires an individualized approach, taking into account several factors:
-
Therapeutic balance: Balancing treatment efficacy, cost, adverse effects, and the animal’s quality of life is essential.
-
Realistic goals: While complete clinical healing remains the theoretical goal, clinical improvement with preserved quality of life can be an acceptable goal in some cases.
-
Therapeutic adaptability: Regular evaluation of treatment response and adjustment of protocols based on this response are crucial for optimizing management.
-
Multimodal approach: Combining different therapeutic modalities (immunomodulatory drugs, local care, pain management, nutritional approach) often offers the best chances of success.
-
Contextual considerations: Financial constraints, the owner’s ability to administer treatments and perform necessary care, as well as potential comorbidities must be integrated into the therapeutic strategy.
Conclusion
Canine perianal fistulas represent a complex dermatological condition with immune-mediated pathogenesis, with particularly high prevalence in German Shepherds. This potentially debilitating pathology can have a major impact on the quality of life of affected animals and requires rapid and appropriate management.
Understanding of this disease has evolved considerably over decades, with a paradigm shift from a primarily anatomical and surgical view to an immune-mediated and medical approach. Immunomodulatory therapies, especially cyclosporine A, are now the cornerstone of treatment, allowing significant healing rates.
Systematic analysis of the scientific literature has made it possible to establish consensus recommendations to guide clinicians in their therapeutic choices. These recommendations highlight the efficacy of cyclosporine A at adapted doses (minimum 5 mg/kg/day), the limited but complementary role of corticotherapy, and the potential interest of certain emerging therapies such as oclacitinib or mesenchymal stem cells.
Surgery, once considered the reference treatment, is now reserved for refractory cases or as a complement after medical stabilization. The dietary approach, although still lacking solid scientific evidence, is often integrated into the overall therapeutic strategy.
The parallels established with human pathologies such as hidradenitis suppurativa or fistulizing Crohn’s disease open interesting perspectives for translational research and the development of new targeted therapeutic approaches.
The ultimate goal of management remains the improvement of the animal’s quality of life, which can sometimes be achieved without complete healing of lesions. An individualized approach, taking into account the specificities of each patient, practical constraints, and owner expectations, remains essential to optimize the chances of long-term therapeutic success.
Frequently Asked Questions
1. Does the initial severity of lesions influence prognosis and treatment response?
Studies have not shown a significant correlation between the initial severity of lesions and treatment response, particularly with cyclosporine. However, early diagnosis and prompt initiation of appropriate treatment generally improve the prognosis. Chronic cases with established fibrosis may be more refractory to therapy.
2. How to manage recurrences after treatment discontinuation?
In case of recurrence after treatment discontinuation, resuming the initially effective protocol is generally recommended. Once remission is achieved, a more gradual tapering may be considered, or maintenance therapy at the minimum effective dose (e.g., reduced-dose cyclosporine or topical tacrolimus) may be continued long-term. Identification and management of potential triggering factors, such as food allergies, are also important.
3. Do environmental factors influence the progression of perianal fistulas?
Although not specifically studied, certain environmental factors appear to influence disease progression. Perianal hygiene, stool consistency (influenced by diet), stress, and physical activity can all impact healing and the animal’s comfort. A clean environment and regular local care contribute positively to the management of the condition.
4. What are the risks of long-term side effects of immunosuppressive treatments?
Long-term immunosuppressive treatments carry several risks:
- For cyclosporine: digestive disorders, gingival hyperplasia, hirsutism, increased risk of opportunistic infections, and potential development of neoplasia (rare)
- For corticosteroids: iatrogenic Cushing’s syndrome, diabetes mellitus, skin atrophy, immunosuppression, risk of secondary infections
- For azathioprine: myelosuppression, hepatotoxicity, pancreatitis
Regular monitoring including clinical examination, blood tests, and dosage adjustments is essential to minimize these risks.
5. Are there ways to predict which German Shepherds will develop this condition?
Currently, there is no validated predictive test to definitively identify German Shepherds at risk of developing perianal fistulas. However, certain risk factors have been identified:
- Dogs homozygous for the DLA-DRB1*00101 allele may develop the disease earlier
- A family history of perianal fistulas appears to increase the risk
- Certain lineages seem to be more affected than others
Further research on genetic and immunological markers could lead to the development of predictive tests in the future, paving the way for preventive strategies in at-risk individuals.