Dermatophytosis, a fungal infection of keratinised tissues, remains a common clinical entity in veterinary medicine. Its polymorphic presentation, coupled with the diversity of aetiological agents and inherent limitations of each diagnostic tool, can sometimes turn its identification into a genuine challenge.
At the recent NAVDF congress held in Orlando last April, Professor Ross Bond, who practices at the Royal Veterinary College in London, provided an in-depth analysis of the epidemiological, clinical, and diagnostic aspects of dermatophytosis, highlighting pitfalls to avoid and offering perspectives for an informed approach, essential for optimal management.
1. Introduction: The Inherent Complexity of Dermatophytes
Dermatophytosis, although familiar to practitioners, hides a complexity that deserves sustained attention. Caused by keratinophilic fungi, mainly from the family Arthrodermataceae, this condition affects hair, claws, and the stratum corneum [1]. If the genera Microsporum et Trichophyton have historically been the most incriminated in animals, fungal taxonomy is constantly evolving. The advent of molecular mycology has thus revealed new genera, such as Nannizzia et Arthroderma, whose mention on laboratory reports may become more frequent [2]. Epidermophyton, for its part, remains a rarity in animal pathology, as it is almost exclusively human in tropism. Faced with this etiological diversity and a kaleidoscope of clinical manifestations, how can the veterinarian confidently steer towards an accurate diagnosis?
2. Epidemiology and Spectrum of Dermatophytes
Understanding the ecology and distribution of dermatophytes is a crucial first step in grasping their pathogenicity.
2.1. Ecological Classification: A Fundamental Triad
Traditional ecological classification distinguishes three major categories of dermatophytes, whose knowledge guides clinical suspicion and the search for the source of infection:
-
Zoophilic : These species, like the paradigmatic Microsporum canis (primarily in cats), are adapted to animals and transmit easily among them. Their identification often involves an investigation of the environment and fellow animals.
-
Geophilic : These fungi, of which Microsporum gypseum is a typical representative, find their reservoir in the soil where they degrade keratin. Contact with contaminated soil is therefore the main mode of transmission.
-
Anthropophilic : Specifically adapted to human hosts, these dermatophytes, such as Trichophyton rubrum, are less frequently the cause of reverse zoonoses (from humans to animals), but this possibility should not be disregarded.
2.2. Geographical Distribution and Host Specificity: Variations to Consider
The prevalence of different species of dermatophytes is not uniform, significantly varying according to geographical region and the animal species involved.
-
In cats, dominates worldwide [3]., Microsporum canis In cattle,
-
Chez les is the major aetiological agent of ringworm, with a global distribution., is the major aetiological agent of ringworm, with a global distribution. est l’agent étiologique majeur de la teigne, avec une distribution également globale.
-
Chez les Horses,, Trichophyton equinum is frequently isolated, but it is noteworthy that strain variations exist, such as the distinct one identified in Australia [4].
2.2.1. The Case of Dogs: A Nuanced Epidemiological Picture
The epidemiology of canine dermatophytosis particularly illustrates this geographical variability:
-
Au In the UK, long-term studies, notably a survey spanning 35 years in Bristol analysing 475 isolates, highlighted the predominance of M. canis (about two-thirds of cases) followed by Trichophyton mentagrophytes (about a quarter) [5]. A more recent follow-up study over 26 years in the same area confirmed this trend, with figures of 42% for M. canis and 38% for T. mentagrophytes [6].
-
Aux In the United States, a study conducted at Louisiana State University in 1991 reported 43% of M. canis and 11% of T. mentagrophytes [7]. Une enquête plus ancienne du CDC, datant de 1958 et portant sur 368 isolements issus de 35 états, montrait une proportion de 69% pour M. canis and 11% for T. mentagrophytes [8].
It thus appears that T. mentagrophytes plays a proportionally larger role in dogs in the UK than in the US, even though M. canis remains a key pathogen in both regions. What environmental or host-related factors might explain these differences?
2.2.2. Other Notable Dermatophytes and Their Ecological Reservoirs
Vigilance is also required for other less common species whose identification may have clinical and epidemiological implications:
-
Trichophyton erinacei : This agent, responsible for “hedgehog ringworm”, sees its prevalence directly correlated with the presence of these insectivores.
-
is the major aetiological agent of ringworm, with a global distribution. : Mainly transmitted by cattle, it is rarely diagnosed in dogs in the United States, and only sporadically in the UK.
-
Microsporum persicolor : With voles and mice as its main reservoir, this dermatophyte is uncommon in dogs in the UK and has not been reported in this species in the US, although its presence in North America (e.g., in Ontario, Canada) is confirmed [9].
-
Microsporum gypseum : This geophilic fungus is a major causal agent of dermatophytosis in Louisiana (44% of canine cases) and remains significant in other American studies (18% of cases). In stark contrast, in Bristol, it took 35 years to isolate M. gypseum from only four dogs. This disparity raises the question of the influence of soil-climatic factors, with warmer soils in the US potentially favouring its survival and proliferation.
2.3. Predisposing Factors: Identifying At-Risk Subjects
Certain factors intrinsic to the animal or related to its environment can increase its susceptibility to dermatophytosis:
-
Age : Young animals, whose immune system is still immature, are classically considered more vulnerable to infection by M. canis. Conversely, older animals, if they are better hunters, might be more exposed to dermatophytes transmitted by wildlife.
-
Breed : Racial predispositions have been suggested. Yorkshire Terriers seem more sensitive to M. canis (in the UK), while Jack Russell Terriers may be more inclined to dermatophytoses acquired from wildlife. Long-haired cats, such as Persians, are also frequently cited.
-
Seasonality : While few marked seasonal variations are noted in the UK, an American study has suggested an increased incidence of T. mentagrophytes in the autumn.
3. The Polymorphic Face of Dermatophytosis: Recognising the Signs
Dermatophytosis is a “great imitator”. Its clinical presentation can be so varied that it requires practitioners to maintain a high index of suspicion. As Michael Schaer reminded us, “If you don’t think of it, you won’t find it” [10] – an adage particularly relevant here.
3.1. Classical Presentations: The Warning Signs
Certain manifestations are considered more typical, although not pathognomonic:
-
Chez les is the major aetiological agent of ringworm, with a global distribution. (T. verrucosum): Characteristic lesions, well-circumscribed, alopecic, covered with greyish, thick scales, preferentially located on the head, neck, and ears. These lesions are more common in animals confined indoors during the winter.
-
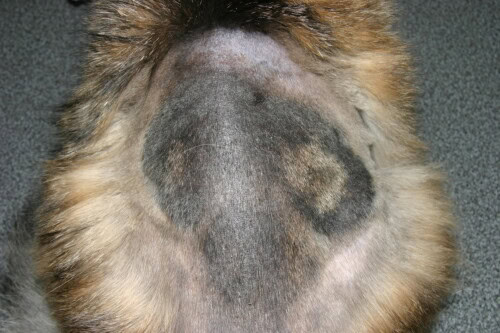
Chez les dogs and cats (M. canis): The classic lesion is a circular, scaly alopecia, sometimes erythematous and crusty, often located on the head. However, less inflammatory forms, confined to an alopecia zone with no marked skin reaction, are also observed.
Il convient de toujours garder à l’esprit l’avertissement de Danny Scott : “Si cela ressemble à la teigne [chez le chien], ce n’en est probablement pas. C’est probablement une folliculite staphylococcique.” [11]. En effet, chez le chien, les lésions annulaires expansives sont plus souvent d’origine bactérienne.
3.2. Atypical Forms and Diagnostic Pitfalls
This is where diagnosis can become a real exercise in subtlety:
-
Folliculitis and Furunculosis of the Muzzle (Sylvatic Dermatophytes) : Frequently associated with T. mentagrophytes ou T. erinacei in dogs that have contact with rodents or hedgehogs (hunting dogs, terriers). These lesions can be unilateral or asymmetrical, with a tendency for central healing and active peripheral inflammation. The nasal planum is typically spared.
-
Nodular Lesions and Plaques (Kerion, Pseudomycetoma) :
-
A Jack Russell Terrier that had explored a rabbit burrow developed an erythematous, scaly-crusty patch on the chest, as well as a distinct nodular lesion on the carpus, both confirmed to be due to T. mentagrophytes.
-
A Doberman presenting with multiple erythematous plaques, initially suspected of having cutaneous lymphoma, was found to have dermatophytosis caused by T. mentagrophytes. What a surprise this unexpected diagnosis was for the clinician!
-
In Persian cats, the pseudomycetoma (or deep keryon) caused by M. canis is a well-described entity, manifesting as nodules, fistulous tracts, and sometimes the expulsion of grains resembling “sulfur grains”.
-
A case reported in Croatia describes a kerion in a cat – a fluctuating, purulent nodule – caused by M. gypseum in association with a staphylococcal superinfection.
-
-
Irregular and Subtle Lesions : A hunting cat presented with discrete alopecia on the nose and erythematous-scaly lesions on the ear pinna caused by T. mentagrophytes, a species uncommon in felines. This underscores the importance of not relying solely on classic fungal species/host associations.
-
Nosocomial Infections : Dermatophyte lesions can develop at sites previously shaved for surgical intervention or the insertion of an intravenous catheter. Rigorous hygiene of clipping equipment, with systematic disinfection between each animal, is therefore an essential preventive measure.
-
Generalised Scaly Form (M. persicolor) : Unlike the majority of other dermatophytes, M. persicolor does not invade the hair shaft but only colonises the stratum corneum. This results in generalised, sometimes intense, desquamation without significant alopecia. An illustrative case involves a dog with a four-year history of generalised scales, finally diagnosed as an infection by M. persicolor.
Very Extensive Form of Feline Dermatophytosis
3.3. Special Cases: Guinea Pigs and Rabbits – The Trap of Asymptomatic Carrier State
These small mammals, particularly those from pet stores or community settings, can be asymptomatic carriers of the T. mentagrophytes complex (frequently identified by molecular techniques as T. benhamiae). This asymptomatic carriage represents a major challenge for infection control.
-
Prevalence of Asymptomatic Carriage :
-
In Belgian pet stores, studies have shown that 3.5% of clinically healthy guinea pigs and 3.8% of rabbits were carriers [12].
-
In Slovakian pet stores, these figures were 10% for guinea pigs and 6% for healthy rabbits [13].
-
A study conducted in Berlin pet stores, using PCR, revealed that although 9% of guinea pigs had clinical lesions, a staggering 90% of all tested guinea pigs were carriers of T. benhamiae [14]. Ces chiffres interpellent quant à la source potentielle d’infection pour d’autres animaux ou pour l’homme.
-
-
Clinical Cases (Rabbits in Spain) : Among rabbits presenting with clinical dermatophyte lesions, 54% of isolates matched T. mentagrophytes and 27% with M. canis [15].
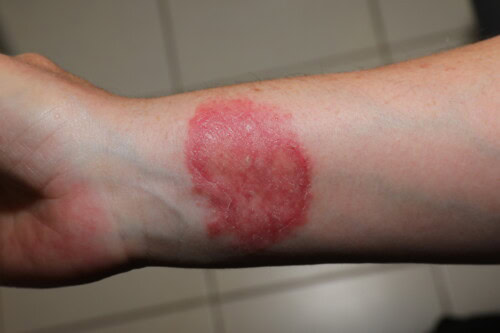
3.4. Dermatophytosis as a Zoonosis: A Constant Concern
It is imperative to remember the zoonotic potential of dermatophytosis. Numerous cases of transmission to humans are documented, affecting both the owners (and their children, as illustrated by a case of transmission from an infected guinea pig) and veterinary staff (a neurology resident having contracted the infection from a canine patient).
Human contamination by a dermatophyte
4. Diagnostic Strategies: Towards a Multimodal Approach
The diagnosis of dermatophytosis does not rely on a single infallible test. A multimodal approach, combining clinical examination with various complementary tests, is key.
4.1. Direct Microscopic Examination (Trichogram): The first examination to perform
This simple and rapid examination can provide valuable clues.
-
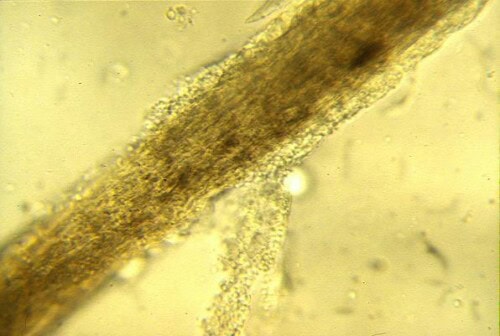
Technique : Hairs should be collected from the active periphery of the lesions, targeting those that appear “cottonlike”, broken, or abnormal. The sample is mounted between a slide and coverslip, usually in a drop of 10-20% KOH (which helps to clarify keratin debris) or simply in mineral oil. The crucial step is to lower the microscope condenser and adjust the diaphragm to increase contrast, as fungal elements are often poorly refractive in white light. One looks for the presence of ectothrix arthrospores (forming a sheath around the hair) or endothrix segmented hyphae (inside the hair shaft).
-
Sensitivity : The Bristol study, comparing microscopy and culture, reported an overall sensitivity of 55%. It was higher for M. canis (58%), often associated with abundant spore production which is easily visible, than for T. mentagrophytes (44%), where hyphae can be more discreet and localised in the scales. It is likely that sensitivity is lower for less experienced practitioners.
-
Pitfalls and Artifacts : Confusion is possible with artifacts such as internal or external hair sheaths, keratin debris, air bubbles, or conidia from saprophytic fungi that might contaminate the skin surface. A good knowledge of fungal morphology is essential.
-
Adjuvant Stains : While not essential if lighting technique is mastered, stains such as “Potash Kwik” (a commercial KOH solution containing ink) or Parker ink can help contrast fungal elements. Calcofluor White is a more sophisticated option (see section 4.5).
Fungal Hair
4.2. Wood’s Lamp: A Classic Tool with Known Limits
The Wood’s lamp remains a useful tool, provided its specifics are known.
-
Principle and Fluorescence : Certain strains of M. canis (but not all) produce, during their metabolism, pteridine, a substance that emits a characteristic apple-green fluorescence when exposed to ultraviolet light at a specific wavelength (around 365 nm). It is important to note that it is the actively infected hairs that fluoresce, not the scales, crusts, or colonies in culture.
-
Specificity and Limits : This examination is mainly relevant for suspected infection by M. canis. The majority of Trichophyton species do not produce this fluorescence. Furthermore, even for M. canis, not all strains are fluorescent.
-
Optimal Examination Conditions : To maximise detection chances, the examination must be conducted in a completely dark room. The lamp should be adequately preheated for several minutes before use. The use of a quality Wood’s lamp, specifically designed for veterinary use and ideally equipped with a magnifying lens, is strongly recommended.
-
Sensitivity : For infections by M. canis, the sensitivity of the Wood’s lamp varies according to studies, generally ranging between 54% and 70% [16]. Thus, a negative result never excludes dermatophytosis.
4.3. Fungal Culture: Identification and Viability
Fungal culture remains a reference method for the precise identification of the species and confirmation of the pathogen’s viability.
-
Sample Collection : Hairs and scales or crusts should be collected from the periphery of the most active lesions. For screening asymptomatic carriers or monitoring therapeutic efficacy, the sterile brush method (such as a McKenzie brush) run over the entire coat, or use of agar plates with a textured side for sampling (like the Derm-Duet), are proven techniques.
-
Culture Media : Sabouraud Dextrose Agar (SDA) is the classical base medium used. Incubation should be carried out at a temperature of 26-27°C and may require up to 4 weeks before a culture can be definitively considered negative.
-
Colony Identification : Identification relies on a combination of criteria:
-
Macroscopic : Colony appearance (colour, texture – cottony, powdery, granular), growth rate, and possible production of a diffusible pigment in the agar or visible on the colony’s reverse.
-
Microscopic : After sampling a portion of the colony (often using transparent adhesive tape applied to the surface then transferred to a slide) and staining (commonly with lactophenol blue), observation of macroconidia and microconidia (shape, size, wall, septation) is critical for species identification. It is crucial to remember that macroconidia are structures produced in vitro (in culture) and are generally not observed on direct animal samples.
-
-
DTM (Dermatophyte Test Medium) : This differential medium is an SDA modified by the addition of a pH indicator (phenol red) and inhibitory agents (such as cycloheximide to inhibit rapidly growing saprophytes, and antibiotics like gentamicin and chlortetracycline to inhibit bacteria). The principle is based on the preferential metabolism of dermatophytes: they initially use the protein sources of the medium, producing alkaline metabolites that change the pH indicator from yellow to red. Saprophytic fungi, on the other hand, tend to first utilize carbohydrates (acidification, so no initial colour change or a shift to yellow), then if the culture is prolonged, they may also metabolize proteins, leading to a late red shift which can be misleading.
-
Interpretation of the DTM : A red colour change early, appearing simultaneously or before the visible growth of the colony (usually within 3 to 7 days), associated with a colony of a compatible appearance (often white, cream, fluffy or powdery), is highly suggestive of a dermatophyte.
-
Limitations of the DTM : Interpretation of DTM in general practice can prove less reliable than in a specialist mycology laboratory. An Israeli study showed an agreement rate of only 80% between clinical readings and those from the laboratory, with more frequent false positives and false negatives in clinical practice [17]. Factors such as an ambient incubation temperature that is too low can delay the colour change. Moreover, prolonged incubation may lead to a red shift by saprophytic fungi, a source of diagnostic error.
-
4.4. Histopathology: When the Lesion is Deep or Atypical
Histopathological examination of skin biopsies can be particularly informative, especially for nodular forms or when other tests are inconclusive.
-
Visualisation of fungal elements : Hyphae can be visualized in the hair shaft, in hair follicles, or sometimes freely in the dermis in cases of furunculosis. Spores may also be observed around the hairs. The use of special stains like PAS (Periodic Acid-Schiff) or GMS (Grocott Methenamine Silver) is often essential to clearly reveal fungal structures, which can be subtle with standard H&E staining.
-
Associated inflammatory patterns :
-
In the case of M. persicolor, the inflammation may be subtle, with primarily lymphocytic infiltration in the squames of the stratum corneum.
-
For most other invasive dermatophytes, a folliculitis (inflammation of the hair follicle), perifolliculitis, or even furunculosis (rupture of the follicle with intense inflammatory reaction in the dermis) can be observed.
-
In cases of kerion or pseudomycetoma, deep inflammatory nodules, often granulomatous or pyogranulomatous, containing fungal elements are typically observed, sometimes in the form of “grains”.
-
4.5. PCR (Polymerase Chain Reaction): A Growing Molecular Tool
PCR enables sensitive detection of fungal DNA from hair and scale samples.
-
Principles and targets : Veterinary PCR panels typically target conserved DNA sequences in dermatophytes, allowing group detection (e.g., Microsporum spp., Trichophyton spp.). More specific primers may then be used to identify certain species of major interest, such as M. canis.
-
Interpretation and nuances :
-
A positive PCR in an animal with compatible clinical signs is highly suggestive of an active infection.
-
In a clinically healthy animal, a positive PCR may indicate asymptomatic carriage or transient environmental contamination of the hair. The detection threshold of PCR is often very low.
-
A major limitation of PCR is its ability to detect fungal DNA from viable or non-viable organisms. Thus, a PCR can remain positive for some time after effective antifungal treatment, simply by detecting DNA from dead spores. It does not necessarily confirm ongoing active infection, and its utility for therapeutic monitoring is debated.
-
-
Comparison with fungal culture : According to mycology expert Patrick Bourdeau, fungal culture, when performed and interpreted by a specialized laboratory, can prove more sensitive than some commercial PCRs. Moreover, culture offers the crucial advantage of allowing precise species identification (essential for epidemiology and source tracing) and confirming the viability of the fungus.
-
Consensus recommendations : The 2017 consensus on feline dermatophytosis from the International Society of Companion Animal Infectious Diseases (ISCAID) recognizes PCR as a useful diagnostic tool but emphasizes that its interpretation must be cautious and contextualized with clinical and epidemiological data [18].
4.6. Fluorescence Microscopy with Calcofluor White: Enhancing Direct Detection
Calcofluor White is a non-specific stain that binds with high affinity to cellulose and chitin, major components of fungal cell walls.
-
Technique : Hair and scale samples are first treated with a KOH solution (to clarify host cellular debris), followed by the addition of a drop of Calcofluor White. After a short incubation time, the sample is examined under a microscope equipped with a UV light source and appropriate filters (generally for near UV excitation and emission in blue or green).
-
Advantages : The fungal elements (hyphae, spores) then appear brightly fluorescent, distinctly standing out from the non-fluorescent background. This technique significantly increases the sensitivity and speed of direct microscopic detection compared to conventional white light examination. It has been reported that using Calcofluor White can nearly double the sensitivity of direct microscopy.
Dermatophytosis is a true crossroads of diagnostic challenges. How do we find our way?
-
The great clinical variability : It necessitates always including dermatophytosis in the differential diagnosis of many skin conditions, even those that do not present the “classic” appearance.
-
The absence of a single infallible “gold standard” : Recent expert consensus highlights that no diagnostic test, taken in isolation, is perfect [18]. A combined approach is often the most fruitful. The combination of Wood’s lamp examination (if an infection with M. canis is suspected) and direct microscopic examination (ideally with Calcofluor White to increase sensitivity) constitutes an excellent first line of investigation in clinical practice.
-
The environmental persistence of spores : The spores of M. canis can remain viable in the environment for periods up to 14 months or more. This longevity significantly complicates infection management, notably the prevention of reinfections and transmission to other animals or humans. Clipping infected animals (if feasible and well-tolerated) may help reduce the environmental load of contaminated hairs and thus spores.
-
The constant zoonotic risk : The infectivity of the animal to humans declines with the establishment of effective antifungal treatment, but precautionary measures (strict hygiene, limiting close contact, especially for children, the elderly, or immunocompromised individuals) must be maintained throughout treatment and until mycological cure is confirmed.
-
Deep nodular forms (kerion or pseudomycetoma) : Their diagnosis can be particularly challenging and often delayed if there is a low initial suspicion of dermatophytosis. In these cases, histopathological examination of deep biopsies, with special stains for fungi, and fungal culture from the biopsied tissue is frequently indispensable.
-
The critical interpretation of test results and their inherent limitations :
-
Direct microscopy : Its sensitivity largely depends on the operator’s experience and the rigour of the technique used (notably the proper adjustment of the condenser).
-
DTM in practice : The risk of misinterpreting the colour change (confusion with the growth of saprophytic fungi in case of late reading or prolonged incubation) is a recognized limitation.
-
PCR : While very sensitive, PCR does not distinguish the DNA of an active infection from the mere presence of viable or non-viable spores. Consequently, a positive PCR on an animal undergoing or recently subjected to treatment does not necessarily mean therapeutic failure.
-
Fungal culture : Regarded as a reference method for species identification and confirmation of pathogen viability, culture can nonetheless be falsely negative if the sample is inadequate, if topical antifungals have been recently applied to lesions, or if laboratory technique is not optimal. It can also be contaminated by rapidly growing saprophytic fungi that may obscure or be confused with dermatophytes.
-
-
The impact of inappropriate corticosteroid use : Administration of glucocorticoids, whether systemically or topically, without a precise etiological diagnosis, can significantly worsen a pre-existing dermatophytosis (dermatophytosis incognito) or obscure clinical signs, making subsequent diagnosis more complex and prolonging treatment.
-
The concept of infectious dose and immunity : A certain quantity of fungal spores is needed to initiate a clinical infection. Animals that develop an effective immune response following an initial infection may show increased resistance to subsequent reinfection by a similar dose of spores.
6. Conclusion and Perspectives: Towards an Informed Practice
Dermatophytosis, by its protean nature and the inherent challenges of its diagnosis, requires the veterinary clinician to maintain constant vigilance and a strategic diagnostic approach. There are no foolproof shortcuts. Recognising the diverse clinical manifestations, understanding the limitations and advantages of each diagnostic tool, and integrating epidemiological data are the pillars of a successful approach.
The future probably lies in optimising and intelligently combining existing techniques. Direct microscopy, enhanced by tools like Calcofluor White, retains a special place for rapid clinical diagnosis. Fungal culture, ideally carried out in specialised laboratories, remains indispensable for precise identification and to assess viability, especially in complex cases or for therapeutic monitoring. PCR, for its part, offers high sensitivity but its interpretation must be cautious and always correlated with the clinical context.
Perhaps we will witness the emergence of rapid molecular tests “at the patient’s bedside” that will combine sensitivity and specificity while informing about viability? Or more standardised diagnostic algorithms integrating the strengths of each method?
While awaiting these advancements, the key message remains: a high clinical suspicion, a judicious selection of tests according to the case, and a critical interpretation of results are essential for optimal management of this fungal condition, for the well-being of our patients and the peace of mind of their owners.
References
[1] Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995 Apr;8(2):240-59.
[2] de Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of Clinical Fungi. 4th ed. Centraalbureau voor Schimmelcultures; 2020.
[3] Moriello KA, Coyner K, Paterson S, Mignon B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017 Jun;28(3):266-e68.
[4] Kano R, Hirai A, Yoshioka N, et al. Arthroderma vanbreuseghemii/Trichophyton mentagrophytes complex isolated from an Australian Cavy. Med Mycol J. 2009;50(3):309-12.
[5] Sparkes AH, Robinson A, MacKay AD, et al. A study of the dermatophytes of dogs and cats in the south of England. J Small Anim Pract. 1993 Oct;34(10):507-13.
[6] Donnely T. Personal communication/unpublished data cited in the presentation. (Note: This would require a formal citation if available, or acknowledgement of the source.)
[7] Miller WH Jr, Scott DW, Wellington JR. Isolation of dermatophytes from the haircoats of normal dogs and cats in the southeastern United States. J Am Anim Hosp Assoc. 1991;27:555-8.
[8] Kaplan W, Georg LK, Ajello L. Recent developments in animal ringworm and their public health implications. Ann N Y Acad Sci. 1958;70:636-49.
[9] Bourdeau P. Dermatophyties des carnivores domestiques : aspects épidémiocliniques et diagnostiques. Point Vét. 2005;36(254):44-51.
[10] Schaer M. Clinical Medicine of the Dog and Cat. 2nd ed. Manson Publishing; 2010. (Note: Attributing this specific quote directly to a publication by Schaer would require verification if not explicitly stated as such in the audio.)
[11] Scott DW, Miller WH Jr, Griffin CE. Muller and Kirk’s Small Animal Dermatology. 7th ed. Elsevier Health Sciences; 2012.
[12] Dekeyser H, Adriaensen C, Mignon B, et al. Asymptomatic carriage of dermatophytes by dogs and cats. Vet Dermatol. 2009 Aug;20(4):253-8.
[13] Čonková E, Kollarova L. Dermatophytes isolated from asymptomatic guinea pigs and rabbits in Slovakia. Mycoses. 2010 Jul;53(4):325-8.
[14] Kraemer A, Mueller RS, Schauer U, et al. Dermatophytes in pet guinea pigs and rabbits. Vet Microbiol. 2012 May 25;157(1-2):208-13.
[15] Cafarchia C, Figueredo LA, Otranto D. Fungal diseases of rabbits. Vet Microbiol. 2013 Nov 15;167(1-2):227-43.
[16] Newbury S. Wood’s lamp examination in small animal practice. Vet Clin North Am Small Anim Pract. 2000 Nov;30(6):1099-105.
[17] Kuzi S, Nivy R, Lavy E, et al. Comparison of real-time PCR and dermatophyte test medium culture for the diagnosis of dermatophytosis in a referral hospital setting. Vet Dermatol. 2016 Feb;27(1):32-e9.
[18] Moriello KA, Coyner K, Paterson S, Mignon B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017 Jun;28(3):266-e68.